当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Anthracene–styrene-substituted m-carborane derivatives: insights into the electronic and structural effects of substituents on photoluminescence
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2020-05-11 , DOI: 10.1039/d0qi00127a Mahdi Chaari 1, 2, 3, 4 , Zsolt Kelemen 1, 2, 3, 4 , Duane Choquesillo-Lazarte 4, 5, 6, 7 , Francesc Teixidor 1, 2, 3, 4 , Clara Viñas 1, 2, 3, 4 , Rosario Núñez 1, 2, 3, 4
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2020-05-11 , DOI: 10.1039/d0qi00127a Mahdi Chaari 1, 2, 3, 4 , Zsolt Kelemen 1, 2, 3, 4 , Duane Choquesillo-Lazarte 4, 5, 6, 7 , Francesc Teixidor 1, 2, 3, 4 , Clara Viñas 1, 2, 3, 4 , Rosario Núñez 1, 2, 3, 4
Affiliation
|
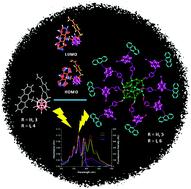
Two anthracenyl–styrenyl-m-carborane triads (one non-iodinated on B, 3, and one iodinated, 4) were synthesized and characterized to be further linked to octavinylsilsesquioxane (OVS) via cross-metathesis, giving rise to the corresponding hybrid materials 5 and 6. The crystal structure of the non-iodinated heterosubstituted-m-carborane 3 was analyzed by X-ray diffraction. Transmission electron microscopy images of pristine OVS and hybrids 5–6 show important differences in the morphology of the particles; whereas OVS forms cubic-like particles, 5–6 have a spherical shape with a broad particle-size distribution. All compounds showed similar vibronic emission spectra in solution, with maxima around 415 nm, assigned to the locally excited state (LE) emission of the anthracene moiety. The similarity with the spectra of the free anthracene (λem = 420 nm) suggested that only small electronic interactions between the anthracene units have taken place, and there is no influence of the iodo or styrene groups on the absorption properties. This is in agreement with the DFT calculations, where calculated oscillator strength corresponding to the transitions from iodo orbitals to the LUMO are weak and could not be observed experimentally. Noticeable, triads 3–4 exhibited exceptional fluorescence quantum yield values of around 100% in solution, that are comparable to those determined for their precursors 1–2, demonstrating that the influence of the styrene group is negligible. Linking these m-carborane derivatives to the OVS led to a significant decrease of quantum yields to 34–45% for 5–6 in solution. Moreover, the PL behavior in the aggregate state was investigated and the spectra of all compounds were very similar, showing emission red-shift with maxima around 455–459 nm. Remarkably, quite high fluorescence quantum yields were determined for 3–4 ((ϕF = 26–31%) and 5–6 (ϕF = 27–36%) in the aggregated state. These data confirm that the m-carborane platform enhances the quantum efficiency of the anthracene in solution, without losing the emission properties in the aggregate state. If this affirmation is associated to other scattered examples on other fluorophores also linked to m-carborane existing in the literature, the former conclusion is reinforced. m-Carborane enhances the fluorescence quantum yield of the free fluorophore, but does not alter the energy of the participating states in the photoluminescence in solution.
中文翻译:

蒽-苯乙烯取代的间-碳氢化合物衍生物:洞察取代基对光致发光的电子和结构效应
两个蒽-styrenyl-米-carborane三单元组(一个非碘化上B,3,和一个碘化,4)被合成和表征将被进一步连接至octavinylsilsesquioxane(OVS)通过交叉复分解反应,产生相应的杂化材料5和6。的非碘化heterosubstituted-晶体结构米-carborane 3通过X射线衍射进行分析。原始OVS和杂化物5–6的透射电子显微镜图像显示出颗粒形态上的重要差异。而OVS形成立方状颗粒,5–6具有球形,粒度分布宽。所有化合物在溶液中均显示出相似的振动电子发射光谱,最大值约为415 nm,这归因于蒽部分的局部激发态(LE)发射。与游离蒽的光谱的相似性(λ EM = 420 nm)的建议,蒽单元之间只有小的电子的相互作用已经发生,并且没有对吸收性能的碘或苯乙烯基团的影响。这与DFT计算是一致的,在DFT计算中,与从碘轨道到LUMO跃迁相对应的计算出的振荡器强度很弱,无法通过实验观察到。引人注目的三合会3-4在溶液中显示出优异的荧光量子产率值,约为100%,与前体1-2的荧光量子产率值相当,这表明苯乙烯基团的影响可忽略不计。连接这些米-carborane衍生物导致的量子产率的降低显著至34-45%,为OVS 5-6在溶液中。此外,对聚集态的PL行为进行了研究,所有化合物的光谱都非常相似,显示出发射红移,最大值在455-459 nm附近。值得注意的是,对于3–4((ϕ F = 26–31%)和5–6(ϕ F= 27–36%)。这些数据证实,该米-carborane平台增强溶液中的蒽的量子效率,而不会在聚合状态失去的发光特性。如果这肯定是关联到上还与其他荧光团等零散的例子米现有文献-carborane,前者结论增强。间-碳硼烷提高了游离荧光团的荧光量子产率,但不改变溶液中光致发光中参与态的能量。
更新日期:2020-05-11
中文翻译:

蒽-苯乙烯取代的间-碳氢化合物衍生物:洞察取代基对光致发光的电子和结构效应
两个蒽-styrenyl-米-carborane三单元组(一个非碘化上B,3,和一个碘化,4)被合成和表征将被进一步连接至octavinylsilsesquioxane(OVS)通过交叉复分解反应,产生相应的杂化材料5和6。的非碘化heterosubstituted-晶体结构米-carborane 3通过X射线衍射进行分析。原始OVS和杂化物5–6的透射电子显微镜图像显示出颗粒形态上的重要差异。而OVS形成立方状颗粒,5–6具有球形,粒度分布宽。所有化合物在溶液中均显示出相似的振动电子发射光谱,最大值约为415 nm,这归因于蒽部分的局部激发态(LE)发射。与游离蒽的光谱的相似性(λ EM = 420 nm)的建议,蒽单元之间只有小的电子的相互作用已经发生,并且没有对吸收性能的碘或苯乙烯基团的影响。这与DFT计算是一致的,在DFT计算中,与从碘轨道到LUMO跃迁相对应的计算出的振荡器强度很弱,无法通过实验观察到。引人注目的三合会3-4在溶液中显示出优异的荧光量子产率值,约为100%,与前体1-2的荧光量子产率值相当,这表明苯乙烯基团的影响可忽略不计。连接这些米-carborane衍生物导致的量子产率的降低显著至34-45%,为OVS 5-6在溶液中。此外,对聚集态的PL行为进行了研究,所有化合物的光谱都非常相似,显示出发射红移,最大值在455-459 nm附近。值得注意的是,对于3–4((ϕ F = 26–31%)和5–6(ϕ F= 27–36%)。这些数据证实,该米-carborane平台增强溶液中的蒽的量子效率,而不会在聚合状态失去的发光特性。如果这肯定是关联到上还与其他荧光团等零散的例子米现有文献-carborane,前者结论增强。间-碳硼烷提高了游离荧光团的荧光量子产率,但不改变溶液中光致发光中参与态的能量。


























 京公网安备 11010802027423号
京公网安备 11010802027423号