Nanomedicine: Nanotechnology, Biology and Medicine ( IF 5.4 ) Pub Date : 2020-05-08 , DOI: 10.1016/j.nano.2020.102215 Jye Yng Teo 1 , Eunkyung Ko 2 , Jiayu Leong 1 , Jiman Hong 3 , Jessie S Jeon 4 , Yi Yan Yang 5 , Hyunjoon Kong 6
|
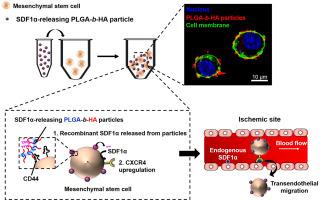
Mesenchymal stem cells are promising medicine for treating diseases and tissue defects because of their innate ability to secrete therapeutic factors. Intravenous delivery of stem cells, although favored for its minimal invasiveness, is often plagued by low cellular engraftment in the target tissue. To this end, this study hypothesizes that in situ activation of cellular expression of CXC chemokine 4 (CXCR4) would significantly improve cellular migration to injured tissue. This hypothesis was examined by tethering the surface of stem cells with poly(D,L-lactide-co-glycolide)-block-hyaluronic acid (HA) particles containing stromal cell-derived factor-1α, a model chemokine to sensitize CXCR4. The HA blocks in the particles enhanced the association rate constant to stem cells by 3.3-fold, and in turn, increased the number of cells expressing CXCR4 receptors. Consequently, these cells displayed 1.2-fold higher transendothelial migration in vitro and 1.7-fold greater trafficking to the ischemic hindlimb of a mouse than that of the untethered cells.
中文翻译:

基质细胞衍生因子-1α 载体与干细胞的表面束缚增强了细胞归巢至缺血肌肉。
间充质干细胞因其天生的分泌治疗因子的能力而成为治疗疾病和组织缺陷的有前途的药物。干细胞的静脉内递送虽然因其最小的侵入性而受到青睐,但通常受到靶组织中细胞植入率低的困扰。为此,本研究假设原位激活 CXC 趋化因子 4 (CXCR4) 的细胞表达将显着改善细胞向受损组织的迁移。这一假设是由干细胞的表面与聚(d,L丙交酯-拴系检查共-乙交酯)-阻断-透明质酸 (HA) 颗粒含有基质细胞衍生因子-1α,这是一种使 CXCR4 敏感的模型趋化因子。颗粒中的 HA 块将与干细胞的结合速率常数提高了 3.3 倍,进而增加了表达 CXCR4 受体的细胞数量。因此,这些细胞在体外表现出高 1.2 倍的跨内皮迁移,向小鼠缺血后肢的运输比未拴系细胞高1.7 倍。

























 京公网安备 11010802027423号
京公网安备 11010802027423号