当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Discovery and Characterization of Peptide Inhibitors for Calcium and Integrin Binding Protein 1.
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-05-08 , DOI: 10.1021/acschembio.0c00144 Ana C Puhl 1 , Jonathan W Bogart 1 , Victoria A Haberman 1 , Jacob E Larson 1 , Andre S Godoy 2 , Jacqueline L Norris-Drouin 1 , Stephanie H Cholensky 1 , Tina M Leisner 3 , Stephen V Frye 1, 4 , Leslie V Parise 3, 4 , Albert A Bowers 1, 4, 5 , Kenneth H Pearce 1, 4
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-05-08 , DOI: 10.1021/acschembio.0c00144 Ana C Puhl 1 , Jonathan W Bogart 1 , Victoria A Haberman 1 , Jacob E Larson 1 , Andre S Godoy 2 , Jacqueline L Norris-Drouin 1 , Stephanie H Cholensky 1 , Tina M Leisner 3 , Stephen V Frye 1, 4 , Leslie V Parise 3, 4 , Albert A Bowers 1, 4, 5 , Kenneth H Pearce 1, 4
Affiliation
|
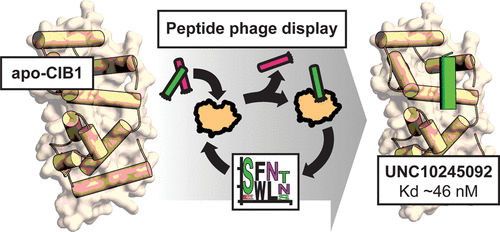
Calcium and integrin binding protein 1 (CIB1) is an EF-hand-containing, small intracellular protein that has recently been implicated in cancer cell survival and proliferation. In particular, CIB1 depletion significantly impairs tumor growth in triple-negative breast cancer (TNBC). Thus, CIB1 is a potentially attractive target for cancer chemotherapy that has yet to be validated by a chemical probe. To produce a probe molecule to the CIB1 helix 10 (H10) pocket and demonstrate that it is a viable target for molecular intervention, we employed random peptide phage display to screen and select CIB1-binding peptides. The top peptide sequence selected, UNC10245092, was produced synthetically, and binding to CIB1 was confirmed by isothermal titration calorimetry (ITC) and a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. Both assays showed that the peptide bound to CIB1 with low nanomolar affinity. CIB1 was cocrystallized with UNC10245092, and the 2.1 Å resolution structure revealed that the peptide binds as an α-helix in the H10 pocket, displacing the CIB1 C-terminal H10 helix and causing conformational changes in H7 and H8. UNC10245092 was further derivatized with a C-terminal Tat-derived cell penetrating peptide (CPP) to demonstrate its effects on TNBC cells in culture, which are consistent with results of CIB1 depletion. These studies provide a first-in-class chemical tool for CIB1 inhibition in cell culture and validate the CIB1 H10 pocket for future probe and drug discovery efforts.
中文翻译:

钙和整联蛋白结合蛋白的肽抑制剂的发现和表征1。
钙和整联蛋白结合蛋白1(CIB1)是一种含有EF手的小细胞内蛋白,最近与癌细胞的存活和增殖有关。特别是,CIB1耗竭显着损害三阴性乳腺癌(TNBC)中的肿瘤生长。因此,CIB1是尚未通过化学探针验证的癌症化学治疗的潜在诱人靶标。为了产生一个探针分子到CIB1螺旋10(H10)口袋并证明它是进行分子干预的可行靶标,我们采用了随机肽噬菌体展示技术来筛选和选择CIB1结合肽。所选的最高肽序列UNC10245092是合成产生的,并通过等温滴定热分析(ITC)和时间分辨的荧光共振能量转移(TR-FRET)分析证实了与CIB1的结合。两种测定均显示该肽以低纳摩尔亲和力与CIB1结合。CIB1与UNC10245092共结晶,2.1分辨率结构显示该肽在H10口袋中以α螺旋的形式结合,取代了CIB1 C端H10螺旋,并导致H7和H8的构象变化。UNC10245092用C末端Tat衍生的细胞穿透肽(CPP)进一步衍生化,以证明其对培养中的TNBC细胞的作用,这与CIB1耗竭的结果一致。这些研究为细胞培养中抑制CIB1提供了一流的化学工具,并验证了CIB1 H10袋的用途,可用于将来的探针和药物发现工作。1Å拆分结构显示该肽在H10口袋中以α螺旋的形式结合,取代了CIB1 C端H10螺旋,并导致H7和H8的构象变化。UNC10245092用C末端Tat衍生的细胞穿透肽(CPP)进一步衍生化,以证明其对培养中的TNBC细胞的作用,这与CIB1耗竭的结果一致。这些研究为细胞培养中抑制CIB1提供了一流的化学工具,并验证了CIB1 H10袋的用途,可用于将来的探针和药物发现工作。1Å拆分结构显示该肽在H10口袋中以α螺旋的形式结合,取代了CIB1 C端H10螺旋,并导致H7和H8的构象变化。UNC10245092用C末端Tat衍生的细胞穿透肽(CPP)进一步衍生化,以证明其对培养中的TNBC细胞的作用,这与CIB1耗竭的结果一致。这些研究为细胞培养中抑制CIB1提供了一流的化学工具,并验证了CIB1 H10袋的用途,可用于将来的探针和药物发现工作。与CIB1耗尽的结果一致。这些研究为细胞培养中抑制CIB1提供了一流的化学工具,并验证了CIB1 H10袋的用途,可用于将来的探针和药物发现工作。与CIB1耗尽的结果一致。这些研究为细胞培养中抑制CIB1提供了一流的化学工具,并验证了CIB1 H10袋的用途,可用于将来的探针和药物发现工作。
更新日期:2020-06-19
中文翻译:

钙和整联蛋白结合蛋白的肽抑制剂的发现和表征1。
钙和整联蛋白结合蛋白1(CIB1)是一种含有EF手的小细胞内蛋白,最近与癌细胞的存活和增殖有关。特别是,CIB1耗竭显着损害三阴性乳腺癌(TNBC)中的肿瘤生长。因此,CIB1是尚未通过化学探针验证的癌症化学治疗的潜在诱人靶标。为了产生一个探针分子到CIB1螺旋10(H10)口袋并证明它是进行分子干预的可行靶标,我们采用了随机肽噬菌体展示技术来筛选和选择CIB1结合肽。所选的最高肽序列UNC10245092是合成产生的,并通过等温滴定热分析(ITC)和时间分辨的荧光共振能量转移(TR-FRET)分析证实了与CIB1的结合。两种测定均显示该肽以低纳摩尔亲和力与CIB1结合。CIB1与UNC10245092共结晶,2.1分辨率结构显示该肽在H10口袋中以α螺旋的形式结合,取代了CIB1 C端H10螺旋,并导致H7和H8的构象变化。UNC10245092用C末端Tat衍生的细胞穿透肽(CPP)进一步衍生化,以证明其对培养中的TNBC细胞的作用,这与CIB1耗竭的结果一致。这些研究为细胞培养中抑制CIB1提供了一流的化学工具,并验证了CIB1 H10袋的用途,可用于将来的探针和药物发现工作。1Å拆分结构显示该肽在H10口袋中以α螺旋的形式结合,取代了CIB1 C端H10螺旋,并导致H7和H8的构象变化。UNC10245092用C末端Tat衍生的细胞穿透肽(CPP)进一步衍生化,以证明其对培养中的TNBC细胞的作用,这与CIB1耗竭的结果一致。这些研究为细胞培养中抑制CIB1提供了一流的化学工具,并验证了CIB1 H10袋的用途,可用于将来的探针和药物发现工作。1Å拆分结构显示该肽在H10口袋中以α螺旋的形式结合,取代了CIB1 C端H10螺旋,并导致H7和H8的构象变化。UNC10245092用C末端Tat衍生的细胞穿透肽(CPP)进一步衍生化,以证明其对培养中的TNBC细胞的作用,这与CIB1耗竭的结果一致。这些研究为细胞培养中抑制CIB1提供了一流的化学工具,并验证了CIB1 H10袋的用途,可用于将来的探针和药物发现工作。与CIB1耗尽的结果一致。这些研究为细胞培养中抑制CIB1提供了一流的化学工具,并验证了CIB1 H10袋的用途,可用于将来的探针和药物发现工作。与CIB1耗尽的结果一致。这些研究为细胞培养中抑制CIB1提供了一流的化学工具,并验证了CIB1 H10袋的用途,可用于将来的探针和药物发现工作。



























 京公网安备 11010802027423号
京公网安备 11010802027423号