当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Peptide Probes for Plasmodium falciparum MyoA Tail Interacting Protein (MTIP): Exploring the Druggability of the Malaria Parasite Motor Complex.
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-05-08 , DOI: 10.1021/acschembio.0c00328 Charlie N Saunders , Ernesto Cota 1 , Jake Baum 1 , Edward W Tate
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-05-08 , DOI: 10.1021/acschembio.0c00328 Charlie N Saunders , Ernesto Cota 1 , Jake Baum 1 , Edward W Tate
Affiliation
|
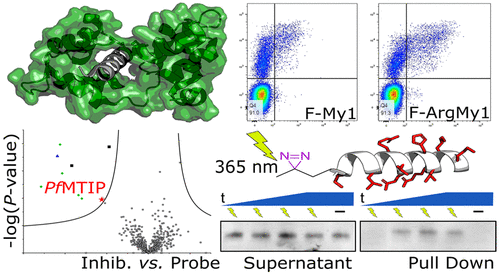
Malaria remains an endemic tropical disease, and the emergence of Plasmodium falciparum parasites resistant to current front-line medicines means that new therapeutic targets are required. The Plasmodium glideosome is a multiprotein complex thought to be essential for efficient host red blood cell invasion. At its core is a myosin motor, Myosin A (MyoA), which provides most of the force required for parasite invasion. Here, we report the design and development of improved peptide-based probes for the anchor point of MyoA, the P. falciparum MyoA tail interacting protein (PfMTIP). These probes combine low nanomolar binding affinity with significantly enhanced cell penetration and demonstrable competitive target engagement with native PfMTIP through a combination of Western blot and chemical proteomics. These results provide new insights into the potential druggability of the MTIP/MyoA interaction and a basis for the future design of inhibitors.
中文翻译:

恶性疟原虫MyoA尾巴相互作用蛋白(MTIP)的肽探针:探索疟疾寄生虫运动复合物的可药物性。
疟疾仍然是一种地方性热带疾病,而对当前一线药物具有抗药性的恶性疟原虫的出现意味着需要新的治疗靶点。该疟原虫glideosome是一个多蛋白复合物认为是有效的主机红细胞入侵至关重要。其核心是肌球蛋白马达,肌球蛋白A(MyoA),它提供了寄生虫入侵所需的大部分力。在这里,我们报告了针对MyoA(恶性疟原虫MyoA尾部相互作用蛋白,Pf MTIP)的锚点的改进的基于肽的探针的设计和开发。这些探针结合了低纳摩尔结合亲和力,显着增强的细胞穿透力以及与天然药物的可证明竞争性靶标结合Pf MTIP通过蛋白质印迹和化学蛋白质组学相结合。这些结果为MTIP / MyoA相互作用的潜在药物作用提供了新的见识,并为抑制剂的未来设计奠定了基础。
更新日期:2020-06-19
中文翻译:

恶性疟原虫MyoA尾巴相互作用蛋白(MTIP)的肽探针:探索疟疾寄生虫运动复合物的可药物性。
疟疾仍然是一种地方性热带疾病,而对当前一线药物具有抗药性的恶性疟原虫的出现意味着需要新的治疗靶点。该疟原虫glideosome是一个多蛋白复合物认为是有效的主机红细胞入侵至关重要。其核心是肌球蛋白马达,肌球蛋白A(MyoA),它提供了寄生虫入侵所需的大部分力。在这里,我们报告了针对MyoA(恶性疟原虫MyoA尾部相互作用蛋白,Pf MTIP)的锚点的改进的基于肽的探针的设计和开发。这些探针结合了低纳摩尔结合亲和力,显着增强的细胞穿透力以及与天然药物的可证明竞争性靶标结合Pf MTIP通过蛋白质印迹和化学蛋白质组学相结合。这些结果为MTIP / MyoA相互作用的潜在药物作用提供了新的见识,并为抑制剂的未来设计奠定了基础。


























 京公网安备 11010802027423号
京公网安备 11010802027423号