当前位置:
X-MOL 学术
›
Fungal Genet. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kilbournase, a protease-associated domain subtilase secreted by the fungal corn pathogen Stenocarpella maydis.
Fungal Genetics and Biology ( IF 3 ) Pub Date : 2020-05-06 , DOI: 10.1016/j.fgb.2020.103399 Todd A Naumann 1 , Michael J Naldrett 2 , Neil P J Price 3
Fungal Genetics and Biology ( IF 3 ) Pub Date : 2020-05-06 , DOI: 10.1016/j.fgb.2020.103399 Todd A Naumann 1 , Michael J Naldrett 2 , Neil P J Price 3
Affiliation
|
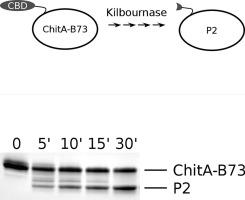
Subtilases are a large family of serine proteases that occur throughout biology. A small subset contain protease-associated (PA) domains that are structurally separate from but encoded within the active site. In bacteria, subtilase PA domains function to recruit specific protein substrates. Here we demonstrate that a protease secreted by the fungal corn pathogen Stenocarpella maydis, which truncates corn ChitA chitinase, is a PA domain subtilase. Protease was purified from S. maydis cultures and tryptic peptides were analyzed by LC-MS/MS. Ions were mapped to two predicted PA domain subtilases. Yeast strains were engineered to express each protease. One failed to produce recombinant protein while the other secreted protease that truncated ChitA. This protease, that we named kilbournase, was purified and characterized. It cleaved multiple peptide bonds in the amino-terminal chitin binding domain of ChitA while leaving the catalytic domain intact. Kilbournase was more active on the ChitA-B73 alloform compared to ChitA-LH82 and did not cleave AtChitIV3, a homolog from Arabidopsis thaliana, indicating a high level of specificity. Truncation of corn ChitA by kilbournase resembles truncation of human C5a by Streptococcus pyogenes ScpA, arguing that PA domain proteases in bacteria and fungi may commonly target specific host proteins.
中文翻译:

Kilbournase,一种蛋白酶相关结构域枯草杆菌酶,由真菌玉米病原体 Stenocarpella maydis 分泌。
Subtilases 是一个大家族的丝氨酸蛋白酶,存在于整个生物学中。一小部分包含蛋白酶相关 (PA) 域,这些域在结构上与活性位点分离但在活性位点内编码。在细菌中,枯草杆菌酶 PA 结构域的功能是募集特定的蛋白质底物。在这里,我们证明了由真菌玉米病原体 Stenocarpella maydis 分泌的蛋白酶,它截断了玉米 ChitA 几丁质酶,是一种 PA 结构域枯草杆菌酶。从 S. maydis 培养物中纯化蛋白酶,并通过 LC-MS/MS 分析胰蛋白酶肽。离子被映射到两个预测的 PA 结构域枯草杆菌酶。酵母菌株被设计为表达每种蛋白酶。一种未能产生重组蛋白,而另一种则分泌截断 ChitA 的蛋白酶。我们将这种蛋白酶命名为 kilbournase,经过纯化和表征。它切割了 ChitA 氨基末端几丁质结合域中的多个肽键,同时保持催化域完整。与 ChitA-LH82 相比,Kilbournase 对 ChitA-B73 同种异体的活性更高,并且不会切割 AtChitIV3(一种来自拟南芥的同源物),表明具有高度的特异性。kilbournase 对玉米 ChitA 的截断类似于化脓性链球菌 ScpA 对人 C5a 的截断,认为细菌和真菌中的 PA 结构域蛋白酶可能通常靶向特定的宿主蛋白。
更新日期:2020-05-06
中文翻译:

Kilbournase,一种蛋白酶相关结构域枯草杆菌酶,由真菌玉米病原体 Stenocarpella maydis 分泌。
Subtilases 是一个大家族的丝氨酸蛋白酶,存在于整个生物学中。一小部分包含蛋白酶相关 (PA) 域,这些域在结构上与活性位点分离但在活性位点内编码。在细菌中,枯草杆菌酶 PA 结构域的功能是募集特定的蛋白质底物。在这里,我们证明了由真菌玉米病原体 Stenocarpella maydis 分泌的蛋白酶,它截断了玉米 ChitA 几丁质酶,是一种 PA 结构域枯草杆菌酶。从 S. maydis 培养物中纯化蛋白酶,并通过 LC-MS/MS 分析胰蛋白酶肽。离子被映射到两个预测的 PA 结构域枯草杆菌酶。酵母菌株被设计为表达每种蛋白酶。一种未能产生重组蛋白,而另一种则分泌截断 ChitA 的蛋白酶。我们将这种蛋白酶命名为 kilbournase,经过纯化和表征。它切割了 ChitA 氨基末端几丁质结合域中的多个肽键,同时保持催化域完整。与 ChitA-LH82 相比,Kilbournase 对 ChitA-B73 同种异体的活性更高,并且不会切割 AtChitIV3(一种来自拟南芥的同源物),表明具有高度的特异性。kilbournase 对玉米 ChitA 的截断类似于化脓性链球菌 ScpA 对人 C5a 的截断,认为细菌和真菌中的 PA 结构域蛋白酶可能通常靶向特定的宿主蛋白。


























 京公网安备 11010802027423号
京公网安备 11010802027423号