当前位置:
X-MOL 学术
›
Thermochim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The intermolecular interactions of methanol, pyrrole and chloroform in a binary solvent
Thermochimica Acta ( IF 3.5 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.tca.2020.178640 Artem A. Petrov , Aliya R. Fakhurtdinova , Artashes A. Khachatrian , Ilnaz T. Rakipov , Aydar A. Akhmadiyarov , Boris N. Solomonov
Thermochimica Acta ( IF 3.5 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.tca.2020.178640 Artem A. Petrov , Aliya R. Fakhurtdinova , Artashes A. Khachatrian , Ilnaz T. Rakipov , Aydar A. Akhmadiyarov , Boris N. Solomonov
|
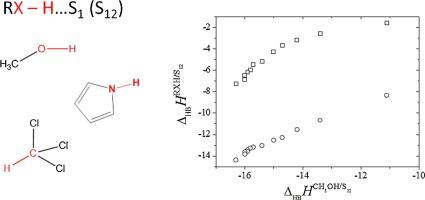
Abstract The work focuses on a thermochemical study of solvation of organic non-electrolytes in various binary mixed solvents. The enthalpies of solution of pyrrole, methanol and chloroform in carbon tetrachloride – pyridine binary mixture were measured at 298.15 K. Data on solution enthalpies of solute – binary solvent for more than 350 systems has been collected. The specific and non-specific terms to the enthalpies of solvation of proton donors have been evaluated for the first time for the mixed solvent using the model previously adapted for individual solvents.
中文翻译:

二元溶剂中甲醇、吡咯和氯仿的分子间相互作用
摘要 工作重点是有机非电解质在各种二元混合溶剂中溶剂化的热化学研究。吡咯、甲醇和氯仿在四氯化碳 - 吡啶二元混合物中的溶液焓在 298.15 K 下测量。已收集了 350 多个系统的溶质 - 二元溶剂的溶液焓数据。使用先前适用于单个溶剂的模型,首次对混合溶剂的质子供体溶剂化焓的特定和非特定项进行了评估。
更新日期:2020-07-01
中文翻译:

二元溶剂中甲醇、吡咯和氯仿的分子间相互作用
摘要 工作重点是有机非电解质在各种二元混合溶剂中溶剂化的热化学研究。吡咯、甲醇和氯仿在四氯化碳 - 吡啶二元混合物中的溶液焓在 298.15 K 下测量。已收集了 350 多个系统的溶质 - 二元溶剂的溶液焓数据。使用先前适用于单个溶剂的模型,首次对混合溶剂的质子供体溶剂化焓的特定和非特定项进行了评估。



























 京公网安备 11010802027423号
京公网安备 11010802027423号