当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Difference of Oxidation Mechanism between Light C3–C4 Alkane and Alkene over Mullite YMn2O5 Oxides’ Catalyst
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-05-06 , DOI: 10.1021/acscatal.0c00703 Tong Zhang 1 , Xiuyao Lang 1 , Anqi Dong 1 , Xiang Wan 1 , Shan Gao 1 , Li Wang 1 , Linxia Wang 1 , Weichao Wang 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-05-06 , DOI: 10.1021/acscatal.0c00703 Tong Zhang 1 , Xiuyao Lang 1 , Anqi Dong 1 , Xiang Wan 1 , Shan Gao 1 , Li Wang 1 , Linxia Wang 1 , Weichao Wang 1
Affiliation
|
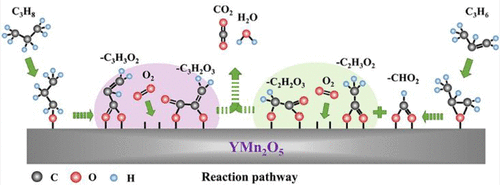
Revealing the catalytic oxidation mechanism of volatile organic compounds (VOCs) is insightful for the development of efficient catalysts. However, because of the complicated interactions and a large number of intermediate species during the reactions, the analysis of the entire reaction mechanism (including the activation modes of reactant molecules and the rate-limiting step) remains a great challenge. Herein, the YMn2O5 mullite catalyst was proposed to demonstrate how to distinguish the deep oxidation difference among C3–C4 alkanes and olefins via combining experiments and theoretical calculations. The YMn2O5 catalyst prepared via the hydrothermal method displayed a superior catalytic behavior with a low T90 temperature (C3H8 at 250 °C; C3H6, C4H10, and C4H8 less than 200 °C) (1000 ppm of C3–C4 and 10% O2 balanced with He, WHSV = 30 000 mL/g·h). The catalytic activity remained the same after continuous reaction for 100 h at 275 °C for each reactant. Overall, the YMn2O5 mullite catalyst exhibits excellent durability with no activity declines for 400 h. Combined with TPD, DRIFTS, XPS, and DFT analysis, surface oxygen species were found to be active for the oxidation. Owing to the difference of the HOMO induced partial charge distributions between alkanes and alkenes, the dehydrogenation of the end-site C–H of propane is the first step prior to the crucial conversion of acrylate over surface labile oxygen in an octahedral ligand unit. For propene oxidation, the C═C double bond is preferentially attacked by two surface oxygen atoms belonging to the octahedral and pyramid ligand units with the crucial step of acetate decomposition. These findings provide insights into the oxide catalyst design toward the complicated VOCs oxidation from a fundamental point of view.
中文翻译:

莫来石YMn 2 O 5氧化物催化剂上轻质C3-C4烷烃与烯烃氧化机理的差异
揭示挥发性有机化合物(VOC)的催化氧化机理对于开发高效催化剂具有深刻的见解。然而,由于反应过程中相互作用复杂,中间产物种类繁多,因此整个反应机理的分析(包括反应物分子的活化方式和限速步骤)仍然是一个很大的挑战。本文中,提出了YMn 2 O 5莫来石催化剂,以证明如何通过结合实验和理论计算来区分C3-C4烷烃和烯烃之间的深层氧化差异。通过水热法制备的YMn 2 O 5催化剂表现出优异的催化性能,且T 90低温度(250°C时的C 3 H 8; C 3 H 6,C 4 H 10和C 4 H 8低于200°C)(1000 ppm的C3-C4和10%O 2与He平衡,WHSV = 30 000 mL / g·h)。每种反应物在275°C下连续反应100小时后,催化活性保持不变。总体而言,YMn 2 O 5莫来石催化剂表现出优异的耐久性,并且在400小时内没有活性下降。结合TPD,DRIFTS,XPS和DFT分析,发现表面氧对氧化具有活性。由于HOMO诱导的烷烃和烯烃之间的部分电荷分布的差异,丙烷的末端位CH脱氢是在八面体配体单元中丙烯酸酯在表面不稳定的氧上进行关键转化之前的第一步。对于丙烯氧化,在乙酸酯分解的关键步骤中,C═C双键优先受到属于八面体和金字塔配体单元的两个表面氧原子的攻击。这些发现从基本的角度提供了对复杂VOC氧化的氧化物催化剂设计的见解。
更新日期:2020-07-02
中文翻译:

莫来石YMn 2 O 5氧化物催化剂上轻质C3-C4烷烃与烯烃氧化机理的差异
揭示挥发性有机化合物(VOC)的催化氧化机理对于开发高效催化剂具有深刻的见解。然而,由于反应过程中相互作用复杂,中间产物种类繁多,因此整个反应机理的分析(包括反应物分子的活化方式和限速步骤)仍然是一个很大的挑战。本文中,提出了YMn 2 O 5莫来石催化剂,以证明如何通过结合实验和理论计算来区分C3-C4烷烃和烯烃之间的深层氧化差异。通过水热法制备的YMn 2 O 5催化剂表现出优异的催化性能,且T 90低温度(250°C时的C 3 H 8; C 3 H 6,C 4 H 10和C 4 H 8低于200°C)(1000 ppm的C3-C4和10%O 2与He平衡,WHSV = 30 000 mL / g·h)。每种反应物在275°C下连续反应100小时后,催化活性保持不变。总体而言,YMn 2 O 5莫来石催化剂表现出优异的耐久性,并且在400小时内没有活性下降。结合TPD,DRIFTS,XPS和DFT分析,发现表面氧对氧化具有活性。由于HOMO诱导的烷烃和烯烃之间的部分电荷分布的差异,丙烷的末端位CH脱氢是在八面体配体单元中丙烯酸酯在表面不稳定的氧上进行关键转化之前的第一步。对于丙烯氧化,在乙酸酯分解的关键步骤中,C═C双键优先受到属于八面体和金字塔配体单元的两个表面氧原子的攻击。这些发现从基本的角度提供了对复杂VOC氧化的氧化物催化剂设计的见解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号