当前位置:
X-MOL 学术
›
Small Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Single‐Molecule Interaction of Peptides with a Biological Nanopore for Identification of Protease Activity
Small Methods ( IF 12.4 ) Pub Date : 2020-05-05 , DOI: 10.1002/smtd.201900892 Ke Sun 1 , Yuan Ju 1 , Chuan Chen 1 , Peng Zhang 1 , Erica Sawyer 1, 2 , Youfu Luo 1 , Jia Geng 1
Small Methods ( IF 12.4 ) Pub Date : 2020-05-05 , DOI: 10.1002/smtd.201900892 Ke Sun 1 , Yuan Ju 1 , Chuan Chen 1 , Peng Zhang 1 , Erica Sawyer 1, 2 , Youfu Luo 1 , Jia Geng 1
Affiliation
|
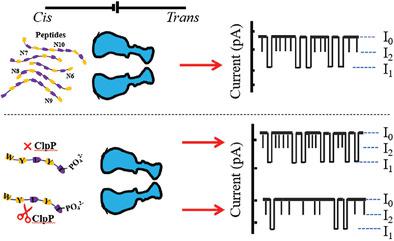
The facile and sensitive detection of peptides is essential for drug screening, pathogen detection, and protein sequencing. There are still challenges for the real‐time single‐molecule sensing and detection of peptides due to their versatile shape, structure, and charges brought by amino acids. Nanopore sensing is an emerging technology for sensing of biomolecules including DNA, RNA, and proteins. In this study, the interaction between peptides of different lengths (N6–N10) and charges with an engineered Mycobacterium smegmatis porin A nanopore are systematically studied, and two types blockage events can be identified by quantifying their dwell times and amplitude of blockades. The findings are further applied to the label‐free and real‐time quantification of protease activity of caseinolytic protease P at nanomolar concentration in 14 min. The protease activity with inhibitor can also be monitored real time by nanopore assay. In summary, this nanopore‐based sensing platform shows promising capacity for peptide detection, protease activities assay, and inhibitor screening.
中文翻译:

肽与生物纳米孔的单分子相互作用,用于鉴定蛋白酶活性
简便,灵敏的肽段检测对于药物筛选,病原体检测和蛋白质测序至关重要。由于氨基酸的多功能形状,结构和电荷,对肽进行实时单分子传感和检测仍然存在挑战。纳米孔感测是一种新兴的技术,用于感测包括DNA,RNA和蛋白质在内的生物分子。在这项研究中,系统地研究了不同长度的肽段(N6-N10)和工程改造的耻垢分枝杆菌孔蛋白A纳米电荷之间的相互作用,并且可以通过量化其停留时间和封锁幅度来鉴定两种类型的封锁事件。这些发现可进一步应用于纳摩尔浓度下14分钟内酪蛋白水解蛋白酶P的蛋白酶活性的无标记和实时定量分析。具有抑制剂的蛋白酶活性也可以通过纳米孔测定法实时监测。总而言之,这个基于纳米孔的传感平台显示出有希望的肽检测,蛋白酶活性测定和抑制剂筛选能力。
更新日期:2020-05-05
中文翻译:

肽与生物纳米孔的单分子相互作用,用于鉴定蛋白酶活性
简便,灵敏的肽段检测对于药物筛选,病原体检测和蛋白质测序至关重要。由于氨基酸的多功能形状,结构和电荷,对肽进行实时单分子传感和检测仍然存在挑战。纳米孔感测是一种新兴的技术,用于感测包括DNA,RNA和蛋白质在内的生物分子。在这项研究中,系统地研究了不同长度的肽段(N6-N10)和工程改造的耻垢分枝杆菌孔蛋白A纳米电荷之间的相互作用,并且可以通过量化其停留时间和封锁幅度来鉴定两种类型的封锁事件。这些发现可进一步应用于纳摩尔浓度下14分钟内酪蛋白水解蛋白酶P的蛋白酶活性的无标记和实时定量分析。具有抑制剂的蛋白酶活性也可以通过纳米孔测定法实时监测。总而言之,这个基于纳米孔的传感平台显示出有希望的肽检测,蛋白酶活性测定和抑制剂筛选能力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号