Cellular and Molecular Gastroenterology and Hepatology ( IF 7.2 ) Pub Date : 2020-04-29 , DOI: 10.1016/j.jcmgh.2020.04.010 Mikhaïl A Van Herck 1 , Luisa Vonghia 1 , Wilhelmus J Kwanten 1 , Yvon Julé 2 , Thomas Vanwolleghem 1 , Didier G Ebo 3 , Peter P Michielsen 1 , Joris G De Man 4 , Lucio Gama 5 , Benedicte Y De Winter 4 , Sven M Francque 1
|
Background & Aims
Nonalcoholic steatohepatitis (NASH) is a multisystem condition, implicating liver and adipose tissue. Although the general involvement of the innate and adaptive immune system has been established, we aimed to define the exact role of the functionally diverse T-cell subsets in NASH pathogenesis through diet reversal and immunologic modulation.
Methods
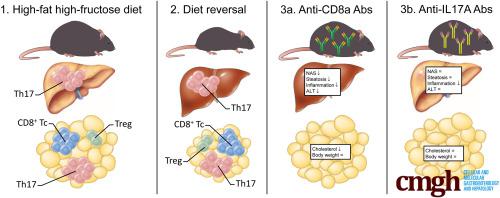
Multiple experimental set-ups were used in 8-week-old C57BL/6J mice, including prolonged high-fat high-fructose diet (HFHFD) feeding, diet reversal from HFHFD to control diet, and administration of anti-CD8a and anti–interleukin 17A antibodies. Plasma alanine aminotransferase, glucose, and lipid levels were determined. Liver and adipose tissue were assessed histologically. Cytotoxic T (Tc), regulatory T, T helper (Th) 1, and Th17 cells were characterized in liver and visceral adipose tissue (VAT) via flow cytometry and RNA analysis.
Results
HFHFD feeding induced the metabolic syndrome and NASH, which coincided with an increase in hepatic Th17, VAT Tc, and VAT Th17 cells, and a decrease in VAT regulatory T cells. Although diet reversal induced a phenotypical metabolic and hepatic normalization, the observed T-cell disruptions persisted. Treatment with anti-CD8a antibodies decreased Tc cell numbers in all investigated tissues and induced a biochemical and histologic attenuation of the HFHFD-induced NASH. Conversely, anti–interleukin 17A antibodies decreased hepatic inflammation without affecting other features of NASH or the metabolic syndrome.
Conclusions
HFHFD feeding induces important immune disruptions in multiple hepatic and VAT T-cell subsets, refractory to diet reversal. In particular, VAT Tc cells are critically involved in NASH pathogenesis, linking adipose tissue inflammation to liver disease.
中文翻译:

饮食逆转和免疫调节显示肝脏和脂肪组织 T 细胞在鼠非酒精性脂肪性肝炎中的关键作用。
背景与目标
非酒精性脂肪性肝炎 (NASH) 是一种多系统疾病,涉及肝脏和脂肪组织。尽管先天性和适应性免疫系统的普遍参与已经建立,但我们的目标是通过饮食逆转和免疫调节来确定功能多样的 T 细胞亚群在 NASH 发病机制中的确切作用。
方法
在 8 周大的 C57BL/6J 小鼠中使用了多种实验设置,包括长期高脂肪高果糖饮食 (HFHFD) 喂养、从 HHFD 逆转饮食以控制饮食,以及施用抗 CD8a 和抗白细胞介素17A 抗体。测定血浆丙氨酸氨基转移酶、葡萄糖和脂质水平。肝脏和脂肪组织进行了组织学评估。通过流式细胞术和 RNA 分析在肝脏和内脏脂肪组织 (VAT) 中表征细胞毒性 T (Tc)、调节性 T、T 辅助 (Th) 1 和 Th17 细胞。
结果
HHFD 喂养诱导了代谢综合征和 NASH,这与肝脏 Th17、VAT Tc 和 VAT Th17 细胞的增加以及 VAT 调节性 T 细胞的减少相吻合。尽管饮食逆转诱导了表型代谢和肝脏正常化,但观察到的 T 细胞破坏仍然存在。用抗 CD8a 抗体治疗减少了所有研究组织中的 Tc 细胞数量,并诱导了 HHFD 诱导的 NASH 的生化和组织学衰减。相反,抗白细胞介素 17A 抗体可减轻肝脏炎症,而不会影响 NASH 或代谢综合征的其他特征。
结论
HHFD 喂养在多个肝脏和增值税 T 细胞亚群中诱导重要的免疫破坏,对饮食逆转无效。特别是,VAT Tc 细胞与 NASH 发病机制密切相关,将脂肪组织炎症与肝脏疾病联系起来。


























 京公网安备 11010802027423号
京公网安备 11010802027423号