当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The crystal structure of the TonB-dependent transporter YncD reveals a positively charged substrate-binding site.
Acta Crystallographica Section D ( IF 2.2 ) Pub Date : 2020-04-27 , DOI: 10.1107/s2059798320004398 Rhys Grinter 1 , Trevor Lithgow 1
Acta Crystallographica Section D ( IF 2.2 ) Pub Date : 2020-04-27 , DOI: 10.1107/s2059798320004398 Rhys Grinter 1 , Trevor Lithgow 1
Affiliation
|
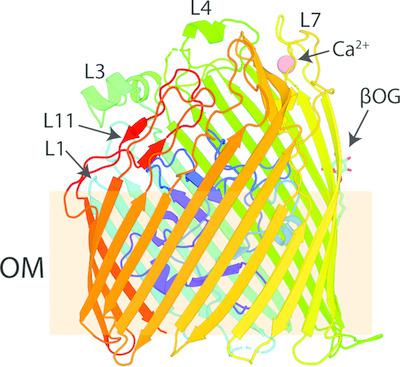
The outer membrane of Gram-negative bacteria is highly impermeable to hydrophilic molecules of larger than 600 Da, protecting these bacteria from toxins present in the environment. In order to transport nutrients across this impermeable membrane, Gram-negative bacteria utilize a diverse family of outer-membrane proteins called TonB-dependent transporters. The majority of the members of this family transport iron-containing substrates. However, it is becoming increasingly clear that TonB-dependent transporters target chemically diverse substrates. In this work, the structure and phylogenetic distribution of the TonB-dependent transporter YncD are investigated. It is shown that while YncD is present in some enteropathogens, including Escherichia coli and Salmonella spp., it is also widespread in Gammaproteobacteria and Betaproteobacteria of environmental origin. The structure of YncD was determined, showing that despite a distant evolutionary relationship, it shares structural features with the ferric citrate transporter FecA, including a compact positively charged substrate-binding site. Despite these shared features, it is shown that YncD does not contribute to the growth of E. coli in pure culture under iron-limiting conditions or with ferric citrate as an iron source. Previous studies of transcriptional regulation in E. coli show that YncD is not induced under iron-limiting conditions and is unresponsive to the ferric uptake regulator (Fur). These observations, combined with the data presented here, suggest that YncD is not responsible for the transport of an iron-containing substrate.
中文翻译:

TonB 依赖性转运蛋白 YncD 的晶体结构揭示了带正电荷的底物结合位点。
革兰氏阴性细菌的外膜对大于 600 Da 的亲水分子高度不可渗透,从而保护这些细菌免受环境中存在的毒素的侵害。为了跨过这种不可渗透的膜运输营养物质,革兰氏阴性细菌利用称为 TonB 依赖性转运蛋白的多种外膜蛋白家族。该家族的大多数成员运输含铁底物。然而,越来越清楚的是,TonB 依赖性转运蛋白以化学多样化的底物为目标。在这项工作中,研究了 TonB 依赖性转运蛋白 YncD 的结构和系统发育分布。结果表明,虽然 YncD 存在于一些肠道病原体中,包括大肠杆菌和沙门氏菌属,但它也广泛存在于环境来源的 Gammaproteobacteria 和 Betaproteobacteria 中。YncD 的结构被确定,表明尽管进化关系遥远,但它与柠檬酸铁转运蛋白 FecA 具有相同的结构特征,包括紧凑的带正电荷的底物结合位点。尽管有这些共同特征,但结果表明,在铁限制条件下或以柠檬酸铁作为铁源的纯培养物中,YncD 不会促进大肠杆菌的生长。先前对大肠杆菌转录调控的研究表明,YncD 在铁限制条件下不会被诱导,并且对铁吸收调节剂 (Fur) 没有反应。这些观察结果与此处提供的数据相结合,表明 YncD 不负责含铁底物的运输。
更新日期:2020-04-27
中文翻译:

TonB 依赖性转运蛋白 YncD 的晶体结构揭示了带正电荷的底物结合位点。
革兰氏阴性细菌的外膜对大于 600 Da 的亲水分子高度不可渗透,从而保护这些细菌免受环境中存在的毒素的侵害。为了跨过这种不可渗透的膜运输营养物质,革兰氏阴性细菌利用称为 TonB 依赖性转运蛋白的多种外膜蛋白家族。该家族的大多数成员运输含铁底物。然而,越来越清楚的是,TonB 依赖性转运蛋白以化学多样化的底物为目标。在这项工作中,研究了 TonB 依赖性转运蛋白 YncD 的结构和系统发育分布。结果表明,虽然 YncD 存在于一些肠道病原体中,包括大肠杆菌和沙门氏菌属,但它也广泛存在于环境来源的 Gammaproteobacteria 和 Betaproteobacteria 中。YncD 的结构被确定,表明尽管进化关系遥远,但它与柠檬酸铁转运蛋白 FecA 具有相同的结构特征,包括紧凑的带正电荷的底物结合位点。尽管有这些共同特征,但结果表明,在铁限制条件下或以柠檬酸铁作为铁源的纯培养物中,YncD 不会促进大肠杆菌的生长。先前对大肠杆菌转录调控的研究表明,YncD 在铁限制条件下不会被诱导,并且对铁吸收调节剂 (Fur) 没有反应。这些观察结果与此处提供的数据相结合,表明 YncD 不负责含铁底物的运输。



























 京公网安备 11010802027423号
京公网安备 11010802027423号