当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, synthesis and SAR study of bridged tricyclic pyrimidinone carboxamides as HIV-1 integrase inhibitors.
Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2020-05-04 , DOI: 10.1016/j.bmc.2020.115541 Manoj Patel 1 , B Narasimhulu Naidu 1 , Ira Dicker 2 , Helen Higley 3 , Zeyu Lin 3 , Brian Terry 3 , Tricia Protack 3 , Mark Krystal 2 , Susan Jenkins 1 , Dawn Parker 1 , Chiradeep Panja 4 , Richard Rampulla 5 , Arvind Mathur 5 , Nicholas A Meanwell 6 , Michael A Walker 7
Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2020-05-04 , DOI: 10.1016/j.bmc.2020.115541 Manoj Patel 1 , B Narasimhulu Naidu 1 , Ira Dicker 2 , Helen Higley 3 , Zeyu Lin 3 , Brian Terry 3 , Tricia Protack 3 , Mark Krystal 2 , Susan Jenkins 1 , Dawn Parker 1 , Chiradeep Panja 4 , Richard Rampulla 5 , Arvind Mathur 5 , Nicholas A Meanwell 6 , Michael A Walker 7
Affiliation
|
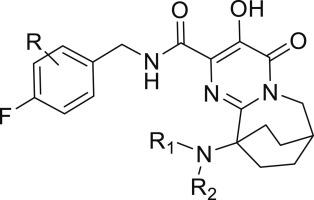
The design, synthesis and structure-activity relationships associated with a series of bridged tricyclic pyrimidinone carboxamides as potent inhibitors of HIV-1 integrase strand transfer are described. Structural modifications to these molecules were made in order to examine the effect on potency towards wild-type and clinically-relevant resistant viruses. The [3.2.2]-bridged tricyclic system was identified as an advantageous chemotype, with representatives exhibiting excellent antiviral activity against both wild-type viruses and the G140S/Q148H resistant virus that arises in response to therapy with raltegravir and elvitegravir.
中文翻译:

作为 HIV-1 整合酶抑制剂的桥连三环嘧啶酮甲酰胺的设计、合成和 SAR 研究。
描述了与一系列桥接三环嘧啶酮甲酰胺作为 HIV-1 整合酶链转移的有效抑制剂相关的设计、合成和结构-活性关系。对这些分子进行结构修饰是为了检查对野生型和临床相关抗性病毒的效力的影响。[3.2.2]-桥接三环系统被确定为一种有利的化学型,其代表对野生型病毒和 G140S/Q148H 抗性病毒均表现出出色的抗病毒活性,这些病毒是响应拉特拉韦和艾维替拉韦治疗而产生的。
更新日期:2020-05-04
中文翻译:

作为 HIV-1 整合酶抑制剂的桥连三环嘧啶酮甲酰胺的设计、合成和 SAR 研究。
描述了与一系列桥接三环嘧啶酮甲酰胺作为 HIV-1 整合酶链转移的有效抑制剂相关的设计、合成和结构-活性关系。对这些分子进行结构修饰是为了检查对野生型和临床相关抗性病毒的效力的影响。[3.2.2]-桥接三环系统被确定为一种有利的化学型,其代表对野生型病毒和 G140S/Q148H 抗性病毒均表现出出色的抗病毒活性,这些病毒是响应拉特拉韦和艾维替拉韦治疗而产生的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号