The Journal of Supercritical Fluids ( IF 3.9 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.supflu.2020.104881 Ali Rasoolzadeh , Sona Raeissi , Alireza Shariati , Cor J. Peters
|
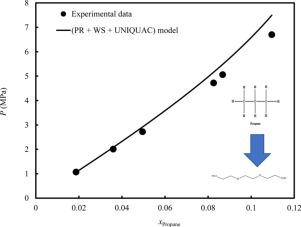
The solubility of propane in TEG was measured via bubble point experiments using the Cailletet equipment. The results indicated that within some ranges of the P-T -x space, this system shows an inverse temperature effect, i.e., an increase of propane solubility with increasing temperatures, contrary to most gas solubilty measurements. Thermodynamic modeling of the phase behavior of propane + TEG system was undertaken using the Peng-Robinson EoS and various mixing rules. The UNIQUAC GE model was used together with the WS and HV mixing rules. The results indicated that the GE mixing rules provides satisfactory results for this system, with the WS mixing rule demonstrating the best results (AARD% of 5.06% in bubble point pressure calculations). The modeling results also indicated that the temperature-dependency of the binary interaction coefficient, , in the vdW mixing rules is very important for accurate results.
中文翻译:

高压丙烷在三甘醇中的溶解度的实验测量和热力学模型
丙烷在TEG中的溶解度是使用Cailletet设备通过泡点实验测量的。结果表明,与大多数气体溶解度测量相反,该系统在PT -x空间的某些范围内显示出反温度效应,即丙烷溶解度随温度升高而增加。使用Peng-Robinson EoS和各种混合规则对丙烷+ TEG系统的相行为进行热力学建模。UNIQUAC G E模型与WS和HV混合规则一起使用。该结果表明,G ^ Ë混合规则为该系统提供了令人满意的结果,而WS混合规则证明了最佳结果(气泡点压力计算中的AARD%为5.06%)。建模结果还表明,二元相互作用系数与温度有关,,在vdW中,混合规则对于获得准确的结果非常重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号