当前位置:
X-MOL 学术
›
Genes Cells
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Multimeric conformation of type III intermediate filaments but not the filamentous conformation exhibits high affinity to lipid bilayers.
Genes to Cells ( IF 2.1 ) Pub Date : 2020-04-03 , DOI: 10.1111/gtc.12768 Beomju Hwang 1 , Hirohiko Ise 2
Genes to Cells ( IF 2.1 ) Pub Date : 2020-04-03 , DOI: 10.1111/gtc.12768 Beomju Hwang 1 , Hirohiko Ise 2
Affiliation
|
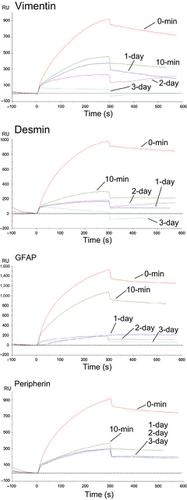
Vimentin, desmin, glial fibrillary acidic protein (GFAP) and peripherin, classified as the type III intermediate filament family, maintain the integrity and architecture of various cell types. Recently, we reported their cell surface expression and binding to multivalent N‐acetylglucosamine‐conjugated polymers. Furthermore, the presence of vimentin on the surface of various cell types including malignant tumor cells and fibroblasts has been demonstrated. Type III intermediate filament proteins are traditionally considered intracellular proteins and do not possess signal peptides for cell membrane recruitment. Therefore, the mechanism of their transport to the cell surface is unclear. In the current study, we aimed to elucidate this mechanism by focusing on the relationship between their multimeric structure and lipid bilayer affinity. Blue native polyacrylamide gel electrophoresis demonstrated that cell surface‐expressed type III intermediate filament proteins formed a multimeric mostly including 4–12‐mers but not filamentous structure. Moreover, surface plasmon resonance analysis revealed that the multimeric structure of these recombinant proteins had high affinity to lipid bilayers, whereas their filament‐like large multimeric structure did not. Our results suggest that type III intermediate filaments are incorporated into the cell membrane through alteration from a filamentous to a multimeric structure.
中文翻译:

III型中间丝的多聚构象但丝状构象不表现出对脂质双层的高亲和力。
波形蛋白,结蛋白,神经胶质纤维酸性蛋白(GFAP)和外周蛋白被分类为III型中间丝家族,可维持各种细胞类型的完整性和结构。最近,我们报道了它们的细胞表面表达以及与多价N-乙酰氨基葡萄糖共轭聚合物的结合。此外,已经证明波形蛋白在包括恶性肿瘤细胞和成纤维细胞在内的各种细胞类型的表面上的存在。III型中间丝蛋白通常被认为是细胞内蛋白,并且不具有用于细胞膜募集的信号肽。因此,它们向细胞表面转运的机理尚不清楚。在当前的研究中,我们旨在通过关注其多聚体结构与脂质双层亲和力之间的关系来阐明这种机制。蓝色天然聚丙烯酰胺凝胶电泳表明,细胞表面表达的III型中间丝蛋白质形成多聚体,主要包括4-12个聚体,但没有丝状结构。此外,表面等离振子共振分析表明,这些重组蛋白的多聚体结构对脂质双层具有高亲和力,而它们的丝状大型多聚体结构则没有。我们的结果表明,III型中间丝通过从丝状变为多聚体结构而掺入细胞膜。表面等离振子共振分析表明,这些重组蛋白的多聚体结构对脂质双层具有很高的亲和力,而其丝状的大的多聚体结构则没有。我们的结果表明,III型中间丝通过从丝状变为多聚体结构而掺入细胞膜。表面等离振子共振分析表明,这些重组蛋白的多聚体结构对脂质双层具有很高的亲和力,而其丝状的大的多聚体结构则没有。我们的结果表明,III型中间丝通过从丝状变为多聚体结构而掺入细胞膜。
更新日期:2020-04-03
中文翻译:

III型中间丝的多聚构象但丝状构象不表现出对脂质双层的高亲和力。
波形蛋白,结蛋白,神经胶质纤维酸性蛋白(GFAP)和外周蛋白被分类为III型中间丝家族,可维持各种细胞类型的完整性和结构。最近,我们报道了它们的细胞表面表达以及与多价N-乙酰氨基葡萄糖共轭聚合物的结合。此外,已经证明波形蛋白在包括恶性肿瘤细胞和成纤维细胞在内的各种细胞类型的表面上的存在。III型中间丝蛋白通常被认为是细胞内蛋白,并且不具有用于细胞膜募集的信号肽。因此,它们向细胞表面转运的机理尚不清楚。在当前的研究中,我们旨在通过关注其多聚体结构与脂质双层亲和力之间的关系来阐明这种机制。蓝色天然聚丙烯酰胺凝胶电泳表明,细胞表面表达的III型中间丝蛋白质形成多聚体,主要包括4-12个聚体,但没有丝状结构。此外,表面等离振子共振分析表明,这些重组蛋白的多聚体结构对脂质双层具有高亲和力,而它们的丝状大型多聚体结构则没有。我们的结果表明,III型中间丝通过从丝状变为多聚体结构而掺入细胞膜。表面等离振子共振分析表明,这些重组蛋白的多聚体结构对脂质双层具有很高的亲和力,而其丝状的大的多聚体结构则没有。我们的结果表明,III型中间丝通过从丝状变为多聚体结构而掺入细胞膜。表面等离振子共振分析表明,这些重组蛋白的多聚体结构对脂质双层具有很高的亲和力,而其丝状的大的多聚体结构则没有。我们的结果表明,III型中间丝通过从丝状变为多聚体结构而掺入细胞膜。



























 京公网安备 11010802027423号
京公网安备 11010802027423号