当前位置:
X-MOL 学术
›
FEBS Open Bio
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interleukin-36α as a potential biomarker for renal tubular damage induced by dietary phosphate load.
FEBS Open Bio ( IF 2.6 ) Pub Date : 2020-04-04 , DOI: 10.1002/2211-5463.12845 Yoshitaka Hirano 1, 2 , Hiroshi Kurosu 1 , Kazuhiro Shiizaki 1 , Yoshitaka Iwazu 1, 3 , Shuichi Tsuruoka 2 , Makoto Kuro-O 1, 4
FEBS Open Bio ( IF 2.6 ) Pub Date : 2020-04-04 , DOI: 10.1002/2211-5463.12845 Yoshitaka Hirano 1, 2 , Hiroshi Kurosu 1 , Kazuhiro Shiizaki 1 , Yoshitaka Iwazu 1, 3 , Shuichi Tsuruoka 2 , Makoto Kuro-O 1, 4
Affiliation
|
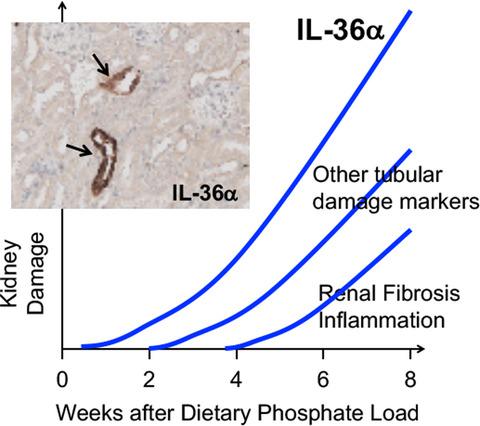
Excessive intake of phosphate has been known to induce renal tubular damage and interstitial inflammation, leading to acute kidney injury or chronic kidney disease in rodents and humans. However, sensitive and early biomarkers for phosphate‐induced kidney damage remain to be identified. Our previous RNA sequencing analysis of renal gene expression identified interleukin‐36α (IL‐36α) as a gene significantly upregulated by dietary phosphate load in mice. To determine the time course and dose dependency of renal IL‐36α expression induced by dietary phosphate load, we placed mice with or without uninephrectomy on a diet containing either 0.35%, 1.0%, 1.5%, or 2.0% inorganic phosphate for 10 days, 4 weeks, or 8 weeks and evaluated renal expression of IL‐36α and other markers of tubular damage and inflammation by quantitative RT‐PCR, immunoblot analysis, and immunohistochemistry. We found that IL‐36α expression was induced in distal convoluted tubules and correlated with phosphate excretion per nephron. The increase in IL‐36α expression was simultaneous with but more robust in amplitude than the increase in tubular damage markers such as Osteopontin and neutrophil gelatinase‐associated lipocalin, preceding the increase in expression of other inflammatory cytokines, including transforming growth factor‐α, interleukin‐1β, and transforming growth factor‐β1. We conclude that IL‐36α serves as a marker that reflects the degree of phosphate load excreted per nephron and of associated kidney damage.
中文翻译:

白介素-36α作为膳食磷酸盐负荷引起的肾小管损害的潜在生物标志物。
已知过量摄入磷酸盐会引起肾小管损伤和间质性炎症,从而导致啮齿动物和人类急性肾损伤或慢性肾脏疾病。但是,对于磷酸盐引起的肾脏损害的敏感和早期生物标志物仍有待确定。我们先前对肾脏基因表达进行的RNA测序分析确定白介素36α(IL‐36α)是小鼠饮食中磷酸盐负荷显着上调的基因。为了确定由饮食磷酸盐负荷引起的肾脏IL-36α表达的时间过程和剂量依赖性,我们将有或没有进行肾切除术的小鼠置于含有0.35%,1.0%,1.5%或2.0%无机磷酸盐的饮食中10天, 4周或8周,并通过定量RT-PCR,免疫印迹分析评估肾脏中IL-36α的表达以及其他肾小管损害和炎症标记,和免疫组化。我们发现,IL-36α表达在远端回旋小管中诱导,并与每个肾单位的磷酸盐排泄相关。与其他损伤性细胞因子(包括转化生长因子-α,白介素)的表达增加相比,IL-36α表达的增加是同时的,但幅度要比骨桥蛋白和中性粒细胞明胶酶相关的脂钙素等肾小管损伤标记的增加强。 -1β和转化生长因子-β1。我们得出的结论是,IL‐36α可作为反映每个肾单位排出的磷酸盐负荷程度和相关肾脏损害的标志物。与其他损伤性细胞因子(包括转化生长因子-α,白介素)的表达增加相比,IL-36α表达的增加是同时的,但幅度要比骨桥蛋白和中性粒细胞明胶酶相关的脂钙素等肾小管损伤标记的增加强。 -1β和转化生长因子-β1。我们得出的结论是,IL‐36α可作为反映每个肾单位排出的磷酸盐负荷程度和相关肾脏损害的标志物。与其他损伤性细胞因子(包括转化生长因子-α,白介素)的表达增加相比,IL-36α表达的增加是同时的,但幅度要比骨桥蛋白和中性粒细胞明胶酶相关的脂钙素等肾小管损伤标记的增加强。 -1β和转化生长因子-β1。我们得出的结论是,IL-36α可作为反映每个肾单位排出的磷酸盐负荷程度和相关肾脏损害的标志物。
更新日期:2020-04-04
中文翻译:

白介素-36α作为膳食磷酸盐负荷引起的肾小管损害的潜在生物标志物。
已知过量摄入磷酸盐会引起肾小管损伤和间质性炎症,从而导致啮齿动物和人类急性肾损伤或慢性肾脏疾病。但是,对于磷酸盐引起的肾脏损害的敏感和早期生物标志物仍有待确定。我们先前对肾脏基因表达进行的RNA测序分析确定白介素36α(IL‐36α)是小鼠饮食中磷酸盐负荷显着上调的基因。为了确定由饮食磷酸盐负荷引起的肾脏IL-36α表达的时间过程和剂量依赖性,我们将有或没有进行肾切除术的小鼠置于含有0.35%,1.0%,1.5%或2.0%无机磷酸盐的饮食中10天, 4周或8周,并通过定量RT-PCR,免疫印迹分析评估肾脏中IL-36α的表达以及其他肾小管损害和炎症标记,和免疫组化。我们发现,IL-36α表达在远端回旋小管中诱导,并与每个肾单位的磷酸盐排泄相关。与其他损伤性细胞因子(包括转化生长因子-α,白介素)的表达增加相比,IL-36α表达的增加是同时的,但幅度要比骨桥蛋白和中性粒细胞明胶酶相关的脂钙素等肾小管损伤标记的增加强。 -1β和转化生长因子-β1。我们得出的结论是,IL‐36α可作为反映每个肾单位排出的磷酸盐负荷程度和相关肾脏损害的标志物。与其他损伤性细胞因子(包括转化生长因子-α,白介素)的表达增加相比,IL-36α表达的增加是同时的,但幅度要比骨桥蛋白和中性粒细胞明胶酶相关的脂钙素等肾小管损伤标记的增加强。 -1β和转化生长因子-β1。我们得出的结论是,IL‐36α可作为反映每个肾单位排出的磷酸盐负荷程度和相关肾脏损害的标志物。与其他损伤性细胞因子(包括转化生长因子-α,白介素)的表达增加相比,IL-36α表达的增加是同时的,但幅度要比骨桥蛋白和中性粒细胞明胶酶相关的脂钙素等肾小管损伤标记的增加强。 -1β和转化生长因子-β1。我们得出的结论是,IL-36α可作为反映每个肾单位排出的磷酸盐负荷程度和相关肾脏损害的标志物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号