当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Palladium/Copper‐catalyzed Oxidation of Aliphatic Terminal Alkenes to Aldehydes Assisted by p‐Benzoquinone
ChemCatChem ( IF 4.5 ) Pub Date : 2020-04-30 , DOI: 10.1002/cctc.202000472 Saki Komori 1 , Yoshiko Yamaguchi 1 , Yuka Murakami 1 , Yasutaka Kataoka 1 , Yasuyuki Ura 1
ChemCatChem ( IF 4.5 ) Pub Date : 2020-04-30 , DOI: 10.1002/cctc.202000472 Saki Komori 1 , Yoshiko Yamaguchi 1 , Yuka Murakami 1 , Yasutaka Kataoka 1 , Yasuyuki Ura 1
Affiliation
|
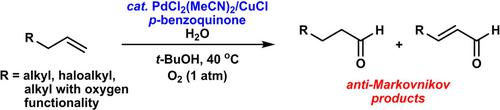
The development of an anti‐Markovnikov Wacker‐type oxidation for simple aliphatic alkenes is a significant challenge. Herein, a variety of aldehydes can be selectively obtained from various unbiased aliphatic terminal alkenes using PdCl2(MeCN)2/CuCl in the presence of p‐benzoquinone (BQ) under mild reaction conditions. Isomerization of the terminal alkene to the internal alkene was suppressed via slow addition of the starting material to the reaction mixture. In addition to the Pd catalyst, CuCl and BQ were essential in order to obtain the anti‐Markovnikov product with high selectivity. Terminal alkenes bearing a halogen substituent afforded their corresponding aldehydes with high anti‐Markovnikov selectivity. The halogen acts as a directing group in the reaction. DFT calculations indicate that a μ‐chloro Pd(II)−Cu(I) bimetallic species with BQ coordinated to Cu is the catalytically active species in the case of a terminal alkene without a directing group.
中文翻译:

对苯醌辅助钯/铜催化脂肪族末端烯烃氧化为醛
为简单的脂肪族烯烃开发反马尔可夫尼科夫·瓦克型氧化是一项重大挑战。在此,在p存在下,可以使用PdCl 2(MeCN)2 / CuCl从各种无偏的脂族末端烯烃中选择性地获得各种醛。苯醌(BQ)在温和的反应条件下。通过将原料缓慢加入反应混合物中,抑制了末端烯烃向内烯烃的异构化。除Pd催化剂外,CuCl和BQ对获得具有高选择性的抗马尔可夫尼可夫产品也是必不可少的。带有卤素取代基的末端烯烃提供了相应的醛,具有很高的抗马尔科夫尼科夫选择性。卤素在反应中充当导向基团。DFT计算表明,在末端烯烃没有导向基团的情况下,具有与铜配位的BQ的μ-氯Pd(II)-Cu(I)双金属物质是催化活性物质。
更新日期:2020-04-30
中文翻译:

对苯醌辅助钯/铜催化脂肪族末端烯烃氧化为醛
为简单的脂肪族烯烃开发反马尔可夫尼科夫·瓦克型氧化是一项重大挑战。在此,在p存在下,可以使用PdCl 2(MeCN)2 / CuCl从各种无偏的脂族末端烯烃中选择性地获得各种醛。苯醌(BQ)在温和的反应条件下。通过将原料缓慢加入反应混合物中,抑制了末端烯烃向内烯烃的异构化。除Pd催化剂外,CuCl和BQ对获得具有高选择性的抗马尔可夫尼可夫产品也是必不可少的。带有卤素取代基的末端烯烃提供了相应的醛,具有很高的抗马尔科夫尼科夫选择性。卤素在反应中充当导向基团。DFT计算表明,在末端烯烃没有导向基团的情况下,具有与铜配位的BQ的μ-氯Pd(II)-Cu(I)双金属物质是催化活性物质。



























 京公网安备 11010802027423号
京公网安备 11010802027423号