当前位置:
X-MOL 学术
›
Mater. Corros.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Corrosion of silver in environment containing halides, pseudohalides, or thiourea
Materials and Corrosion ( IF 1.8 ) Pub Date : 2020-04-14 , DOI: 10.1002/maco.202011665 Jan Švadlena 1 , Eva Voráčová 1 , Jan Stoulil 1
Materials and Corrosion ( IF 1.8 ) Pub Date : 2020-04-14 , DOI: 10.1002/maco.202011665 Jan Švadlena 1 , Eva Voráčová 1 , Jan Stoulil 1
Affiliation
|
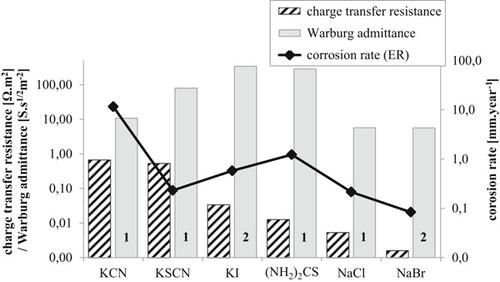
Many studies of silver corrosion have been focused on indoor sulfur‐containing atmospheres. However, significant corrosion damage of silver can also occur in the presence of other corrosion stimulants, such as halides, pseudohalides, or thiourea. These environments pose a specific threat for historical silver objects like daguerreotypes or coins, for example, during incorrect storing or cleaning method. To better characterize the silver corrosion caused by the solutions, electrochemical impedance spectroscopy (EIS) and electrical resistance technique were used. EIS measurements show that the dissolution of silver in tested solutions is mainly controlled by diffusion, suggesting the formation of a layer on the sample surface. For the electrical resistance technique, two different setups with electrical resistance probes were used: in saturated solutions and in atmospheric conditions simulating corrosion under deposits. In the latter setup, the corrosion rates decreased after a certain amount of time which can be explained by the presence of a salt layer (as suggested from EIS results). Regardless of the arrangement, cyanide and thiourea environment resulted in the highest corrosion rates.
中文翻译:

银在含有卤化物,假卤化物或硫脲的环境中的腐蚀
银腐蚀的许多研究都集中在室内含硫气氛中。但是,在存在其他腐蚀刺激剂(例如卤化物,拟卤化物或硫脲)的情况下,银也可能发生严重的腐蚀破坏。例如,在不正确的存储或清洁方法期间,这些环境会对历史古银物品(例如双币型或硬币)构成特殊威胁。为了更好地表征溶液引起的银腐蚀,使用了电化学阻抗谱(EIS)和电阻技术。EIS测量表明,银在测试溶液中的溶解主要受扩散控制,这表明在样品表面形成了一层。对于电阻技术,使用了带有电阻探头的两种不同设置:在饱和溶液中和在大气条件下模拟沉积物下的腐蚀。在后一种设置中,腐蚀速率在一定时间后降低,这可以用盐层的存在来解释(如EIS结果所示)。无论如何布置,氰化物和硫脲环境都会导致最高的腐蚀速率。
更新日期:2020-04-14
中文翻译:

银在含有卤化物,假卤化物或硫脲的环境中的腐蚀
银腐蚀的许多研究都集中在室内含硫气氛中。但是,在存在其他腐蚀刺激剂(例如卤化物,拟卤化物或硫脲)的情况下,银也可能发生严重的腐蚀破坏。例如,在不正确的存储或清洁方法期间,这些环境会对历史古银物品(例如双币型或硬币)构成特殊威胁。为了更好地表征溶液引起的银腐蚀,使用了电化学阻抗谱(EIS)和电阻技术。EIS测量表明,银在测试溶液中的溶解主要受扩散控制,这表明在样品表面形成了一层。对于电阻技术,使用了带有电阻探头的两种不同设置:在饱和溶液中和在大气条件下模拟沉积物下的腐蚀。在后一种设置中,腐蚀速率在一定时间后降低,这可以用盐层的存在来解释(如EIS结果所示)。无论如何布置,氰化物和硫脲环境都会导致最高的腐蚀速率。


























 京公网安备 11010802027423号
京公网安备 11010802027423号