当前位置:
X-MOL 学术
›
Mater. Corros.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Influence of pH on corrosion behavior of carbon steel in simulated cooling water containing scale and corrosion inhibitors
Materials and Corrosion ( IF 1.8 ) Pub Date : 2020-02-25 , DOI: 10.1002/maco.202011516 Jiahui Yao 1 , Honghua Ge 1 , Yi Zhang 1 , Xiaojie Wang 1 , Siyu Xie 1 , Kun Sheng 1 , Xinjing Meng 1 , Yuzeng Zhao 1
Materials and Corrosion ( IF 1.8 ) Pub Date : 2020-02-25 , DOI: 10.1002/maco.202011516 Jiahui Yao 1 , Honghua Ge 1 , Yi Zhang 1 , Xiaojie Wang 1 , Siyu Xie 1 , Kun Sheng 1 , Xinjing Meng 1 , Yuzeng Zhao 1
Affiliation
|
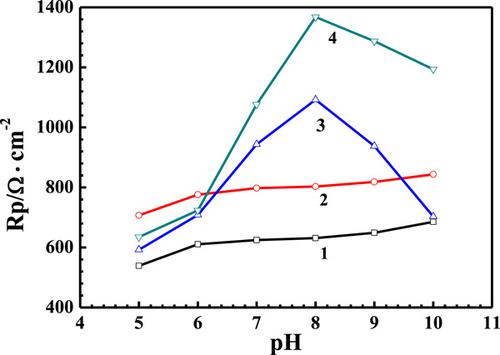
In this paper, the influence of pH on the corrosion behavior of AISI 1020 carbon steel in simulated cooling water was investigated by using electrochemical and surface analysis methods. The results of polarization showed that the corrosion resistance of carbon steel increased with an increase in pH of the simulated water, and the corrosion control process changed from cathodic polarization to anode polarization control. The scale and corrosion inhibitor 2‐phosphonobutane‐1,2,4‐tricarboxylic acid (PBTCA) had a certain anodic corrosion inhibition effect on carbon steel, whereas Zn2+ acted as a cathodic inhibitor for carbon steel in simulated water with pH 7–9. In simulated water containing both PBTCA and Zn2+, a good synergistic corrosion inhibition was found between PBTCA and Zn2+, and their corrosion inhibition effect on carbon steel was the best at pH 8. This was attributed to the formation of Zn(OH)2 precipitate film in the cathode region and the formation of Zn–PBTCA complex film in the anode region at this pH.
中文翻译:

pH对含垢和缓蚀剂的模拟冷却水中碳钢腐蚀行为的影响
本文采用电化学和表面分析方法研究了pH值对模拟冷却水中AISI 1020碳钢腐蚀行为的影响。极化结果表明,碳钢的耐蚀性随模拟水pH值的增加而增加,腐蚀控制过程由阴极极化转变为阳极极化。阻垢剂和缓蚀剂2-膦酰基丁烷1,2,4-三羧酸(PBTCA)对碳钢具有一定的阳极缓蚀作用,而Zn 2+则在pH 7–7的模拟水中充当碳钢的阴极缓蚀剂。 9。在同时含有PBTCA和Zn 2+的模拟水中,发现PBTCA和Zn之间具有良好的协同腐蚀抑制作用2+,其对碳钢的腐蚀抑制作用在pH值为8时最好。这归因于在阴极区域形成Zn(OH)2沉淀膜,在阳极区域形成Zn-PBTCA复合膜。这个pH值。
更新日期:2020-02-25
中文翻译:

pH对含垢和缓蚀剂的模拟冷却水中碳钢腐蚀行为的影响
本文采用电化学和表面分析方法研究了pH值对模拟冷却水中AISI 1020碳钢腐蚀行为的影响。极化结果表明,碳钢的耐蚀性随模拟水pH值的增加而增加,腐蚀控制过程由阴极极化转变为阳极极化。阻垢剂和缓蚀剂2-膦酰基丁烷1,2,4-三羧酸(PBTCA)对碳钢具有一定的阳极缓蚀作用,而Zn 2+则在pH 7–7的模拟水中充当碳钢的阴极缓蚀剂。 9。在同时含有PBTCA和Zn 2+的模拟水中,发现PBTCA和Zn之间具有良好的协同腐蚀抑制作用2+,其对碳钢的腐蚀抑制作用在pH值为8时最好。这归因于在阴极区域形成Zn(OH)2沉淀膜,在阳极区域形成Zn-PBTCA复合膜。这个pH值。


























 京公网安备 11010802027423号
京公网安备 11010802027423号