当前位置:
X-MOL 学术
›
Mater. Corros.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of the temperature of cerium nitrate–NaCl solution on corrosion inhibition of mild steel
Materials and Corrosion ( IF 1.8 ) Pub Date : 2020-01-30 , DOI: 10.1002/maco.201911472 Hichem Boudellioua 1 , Youcef Hamlaoui 2 , Lakhdar Tifouti 1 , Fernando Pedraza 3
Materials and Corrosion ( IF 1.8 ) Pub Date : 2020-01-30 , DOI: 10.1002/maco.201911472 Hichem Boudellioua 1 , Youcef Hamlaoui 2 , Lakhdar Tifouti 1 , Fernando Pedraza 3
Affiliation
|
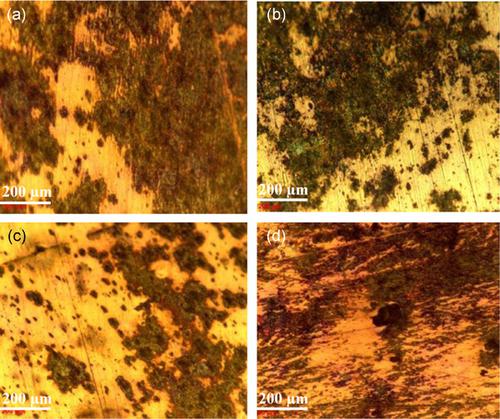
In this study, the effect of temperature on corrosion inhibition is studied in the absence and presence of an optimal concentration of cerium nitrate (600 mg/L) as an inhibitor of mild steel in sodium chloride. Corrosion tests are carried out through electrochemical techniques such as impedance spectroscopy and d.c. polarization measurements. The surface morphology of the films is investigated by optical microscopy, white‐light interferometry, and scanning electronic microscopy, coupled with energy‐dispersive spectroscopy analysis for chemical composition. The results obtained show that the activation energy for the corrosion inhibition process to occur increases in the presence of a cerium nitrate inhibitor. However, the corrosion resistance of mild steel is somewhat lost upon increasing the solution temperature up to 55°C, which leads to more cracked films. The enthalpy and entropy values suggest a mixed mechanism of chemisorption and physisorption inhibition, with a major dominance of physisorption control.
中文翻译:

硝酸铈-氯化钠溶液温度对低碳钢腐蚀抑制的影响
在这项研究中,研究了在不存在和存在最适浓度硝酸铈(600 mg / L)作为氯化钠中低碳钢抑制剂的情况下,温度对腐蚀抑制的影响。腐蚀测试是通过电化学技术进行的,例如阻抗谱和直流极化测量。通过光学显微镜,白光干涉法和扫描电子显微镜以及化学成分的能量分散光谱分析研究了膜的表面形态。获得的结果表明,在硝酸铈抑制剂的存在下,用于发生腐蚀抑制过程的活化能增加。但是,将固溶温度提高到55°C时,软钢的耐腐蚀性会有所降低,这导致更多的破裂的电影。焓值和熵值表明了化学吸附和物理吸附抑制的混合机制,主要是物理吸附控制。
更新日期:2020-01-30
中文翻译:

硝酸铈-氯化钠溶液温度对低碳钢腐蚀抑制的影响
在这项研究中,研究了在不存在和存在最适浓度硝酸铈(600 mg / L)作为氯化钠中低碳钢抑制剂的情况下,温度对腐蚀抑制的影响。腐蚀测试是通过电化学技术进行的,例如阻抗谱和直流极化测量。通过光学显微镜,白光干涉法和扫描电子显微镜以及化学成分的能量分散光谱分析研究了膜的表面形态。获得的结果表明,在硝酸铈抑制剂的存在下,用于发生腐蚀抑制过程的活化能增加。但是,将固溶温度提高到55°C时,软钢的耐腐蚀性会有所降低,这导致更多的破裂的电影。焓值和熵值表明了化学吸附和物理吸附抑制的混合机制,主要是物理吸附控制。


























 京公网安备 11010802027423号
京公网安备 11010802027423号