当前位置:
X-MOL 学术
›
STEM CELLS
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
N-glycosylation controls inflammatory licensing-triggered PD-L1 upregulation in human mesenchymal stromal cells
STEM CELLS ( IF 5.2 ) Pub Date : 2020-05-12 , DOI: 10.1002/stem.3190 Vivien Strauch 1 , Domenica Saul 1 , Mirjeta Berisha 1 , Andreas Mackensen 1 , Dimitrios Mougiakakos 1 , Regina Jitschin 1
STEM CELLS ( IF 5.2 ) Pub Date : 2020-05-12 , DOI: 10.1002/stem.3190 Vivien Strauch 1 , Domenica Saul 1 , Mirjeta Berisha 1 , Andreas Mackensen 1 , Dimitrios Mougiakakos 1 , Regina Jitschin 1
Affiliation
|
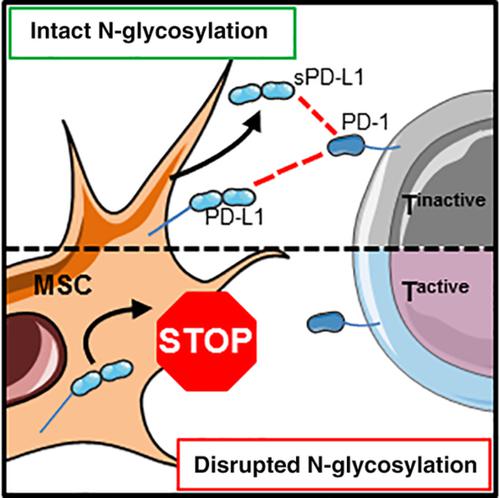
Mesenchymal stromal cells (MSCs) are characterized by their multipotency, regenerative potential, and immunoregulatory properties. Nowadays, MSCs represent a promising cell‐therapeutic option for hyperinflammatory conditions such as graft‐vs‐host disease following allogeneic hematopoietic stem cell transplantation. A better understanding of their biology is a prerequisite for improving their treatment efficacy. Emerging evidence suggests that immunosuppressive properties are not constitutively active in MSCs. Instead, microenvironmental inflammatory stimuli such as the cytokines interferon (IFN)‐γ or tumor necrosis factor (TNF)‐α license MSCs to acquire a tolerance‐promoting phenotype. The immunological checkpoint molecule programmed death‐ligand 1 (PD‐L1) is an important regulator of T‐cell responses. Binding of PD‐L1 to the programmed cell death protein 1 (PD‐1) receptor on T‐cells suppresses their activation, proliferation, and induces apoptosis. Previous studies have revealed that cell surface expression and secretion of PD‐L1 are part of the MSCs' immunomodulatory armamentarium. Here, we report that inflammatory licensing leads to an enhanced PD‐L1 cell surface expression and secretion, which are both accompanied by an increased posttranslational protein N‐glycosylation. These post‐translational modifications have been shown to be critical for key biological processes such as cell trafficking, receptor signaling, and immunohomeostasis. In fact, promoting N‐glycosylation in MSCs yielded increased PD‐L1 levels. We report for the first time that PD‐L1 N‐glycosylation plays a decisive role for its transport to the MSCs' cell surface and its subsequent secretion (in response to proinflammatory trigger). Our data offer insights into a novel regulatory mechanism with the potential to be exploited as a means to foster the immunosuppressive potency of human MSCs.
中文翻译:

N-糖基化控制炎症许可触发的人间充质基质细胞中的 PD-L1 上调
间充质基质细胞 (MSC) 的特点是其多能性、再生潜力和免疫调节特性。如今,间充质干细胞代表了一种有希望的细胞治疗选择,用于治疗异基因造血干细胞移植后的高炎症性疾病,例如移植物抗宿主病。更好地了解它们的生物学是提高它们的治疗效果的先决条件。新出现的证据表明,免疫抑制特性在 MSC 中不具有组成性活性。相反,细胞因子干扰素 (IFN)-γ 或肿瘤坏死因子 (TNF)-α 等微环境炎症刺激允许 MSC 获得耐受促进表型。免疫检查点分子程序性死亡配体 1 (PD-L1) 是 T 细胞反应的重要调节剂。PD-L1 与 T 细胞上的程序性细胞死亡蛋白 1 (PD-1) 受体结合可抑制其活化、增殖并诱导细胞凋亡。先前的研究表明,细胞表面表达和 PD-L1 的分泌是 MSCs 免疫调节武器的一部分。在这里,我们报告炎症许可导致 PD-L1 细胞表面表达和分泌增强,这两者都伴随着翻译后蛋白 N-糖基化的增加。这些翻译后修饰已被证明对细胞运输、受体信号传导和免疫稳态等关键生物过程至关重要。事实上,促进 MSC 中的 N-糖基化会增加 PD-L1 水平。我们首次报道了 PD-L1 N-糖基化对其向 MSC 的转运起决定性作用 细胞表面及其随后的分泌(响应促炎触发)。我们的数据提供了对一种新的调控机制的见解,该机制有可能被用作促进人类 MSCs 免疫抑制效力的手段。
更新日期:2020-05-12
中文翻译:

N-糖基化控制炎症许可触发的人间充质基质细胞中的 PD-L1 上调
间充质基质细胞 (MSC) 的特点是其多能性、再生潜力和免疫调节特性。如今,间充质干细胞代表了一种有希望的细胞治疗选择,用于治疗异基因造血干细胞移植后的高炎症性疾病,例如移植物抗宿主病。更好地了解它们的生物学是提高它们的治疗效果的先决条件。新出现的证据表明,免疫抑制特性在 MSC 中不具有组成性活性。相反,细胞因子干扰素 (IFN)-γ 或肿瘤坏死因子 (TNF)-α 等微环境炎症刺激允许 MSC 获得耐受促进表型。免疫检查点分子程序性死亡配体 1 (PD-L1) 是 T 细胞反应的重要调节剂。PD-L1 与 T 细胞上的程序性细胞死亡蛋白 1 (PD-1) 受体结合可抑制其活化、增殖并诱导细胞凋亡。先前的研究表明,细胞表面表达和 PD-L1 的分泌是 MSCs 免疫调节武器的一部分。在这里,我们报告炎症许可导致 PD-L1 细胞表面表达和分泌增强,这两者都伴随着翻译后蛋白 N-糖基化的增加。这些翻译后修饰已被证明对细胞运输、受体信号传导和免疫稳态等关键生物过程至关重要。事实上,促进 MSC 中的 N-糖基化会增加 PD-L1 水平。我们首次报道了 PD-L1 N-糖基化对其向 MSC 的转运起决定性作用 细胞表面及其随后的分泌(响应促炎触发)。我们的数据提供了对一种新的调控机制的见解,该机制有可能被用作促进人类 MSCs 免疫抑制效力的手段。



























 京公网安备 11010802027423号
京公网安备 11010802027423号