当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Spectroscopic studies, Density Functional Theory calculations, and non‐linear optical properties of binuclear Fe(III), Co(II), Ni(II), Cu(II), and Zn(II) complexes of OONN Schiff base ligand
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2020-04-28 , DOI: 10.1002/jccs.202000024 Hussein Moustafa 1 , Gehad G. Mohamed 1, 2 , Salwa Elramly 3
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2020-04-28 , DOI: 10.1002/jccs.202000024 Hussein Moustafa 1 , Gehad G. Mohamed 1, 2 , Salwa Elramly 3
Affiliation
|
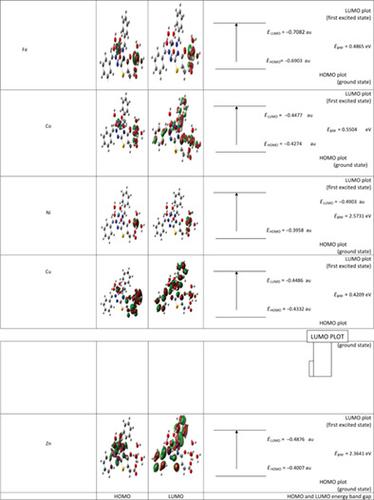
The binuclear complexes of Fe(III), Co(II), Ni(II), Cu(II), and Zn(II) with 2‐[3‐(benzylideneamino)‐2‐(benzylidenehydrazono)‐4‐oxothiazolidin‐5‐yl] acetic acid ligand (HL) were prepared and their stoichiometry was determined by elemental analysis. The stereochemistry of the studied binuclear metal complexes was confirmed by analyzing their infrared spectra, 1H NMR, and magnetic moment. Thermal decomposition studies of the binuclear complexes have been performed to demonstrate the status of water molecules present in these binuclear complexes and their general decomposition pattern. The equilibrium geometry of the ligand and its studied complexes were calculated using density function theory (DFT) calculations at the B3LYP/GENECP level of the theory. The results show that the ligand and its complexes are nonplanar structures as indicated from the values of the dihedral angles. Extent of distortion from regular geometry has been performed and discussed in terms of the values of the angles between the central metals and the coordinated sites. The EHOMO and ELUMO energies of the studied ligand and its complexes are used to calculate the global properties. The nonlinear optical parameters (NLO), anisotropy of the polarizibility (Δα), and the mean first‐order hyperpolarizability (<β>) were calculated. The (<β>) values were compared with Urea as a reference molecule and the results of (<β>) values showed that the ligand and the studied complexes have good NLO behaviors.
中文翻译:

OONN Schiff碱配体的双核Fe(III),Co(II),Ni(II),Cu(II)和Zn(II)配合物的光谱研究,密度泛函理论计算和非线性光学性质
Fe(III),Co(II),Ni(II),Cu(II)和Zn(II)与2- [3-(苄叉氨基)-2-(苄叉肼基)-4-氧噻唑啉酮-5]的双核络合物制备了[yl]乙酸配体(HL),并通过元素分析确定了其化学计量。分析双核金属配合物的红外光谱可确认其立体化学,11 H NMR和磁矩。已经对双核络合物进行了热分解研究,以证明这些双核络合物中存在的水分子的状态及其一般的分解模式。使用密度函数理论(DFT)在该理论的B3LYP / GENECP级别上计算该配体及其研究的络合物的平衡几何。结果表明,如从二面角的值所示,配体及其配合物是非平面结构。关于中心金属和配位部位之间的夹角值,已经进行并讨论了规则几何形变的程度。该Ë HOMO和Ë LUMO研究的配体及其配合物的能量用于计算整体性质。非线性光学参数(NLO),各向异性的polarizibility(Δ的α),且平均第一阶超极化率(< β >)进行了计算。将(< β >)值与作为参考分子的尿素进行比较,(< β >)值的结果表明配体和所研究的配合物具有良好的NLO行为。
更新日期:2020-04-28
中文翻译:

OONN Schiff碱配体的双核Fe(III),Co(II),Ni(II),Cu(II)和Zn(II)配合物的光谱研究,密度泛函理论计算和非线性光学性质
Fe(III),Co(II),Ni(II),Cu(II)和Zn(II)与2- [3-(苄叉氨基)-2-(苄叉肼基)-4-氧噻唑啉酮-5]的双核络合物制备了[yl]乙酸配体(HL),并通过元素分析确定了其化学计量。分析双核金属配合物的红外光谱可确认其立体化学,11 H NMR和磁矩。已经对双核络合物进行了热分解研究,以证明这些双核络合物中存在的水分子的状态及其一般的分解模式。使用密度函数理论(DFT)在该理论的B3LYP / GENECP级别上计算该配体及其研究的络合物的平衡几何。结果表明,如从二面角的值所示,配体及其配合物是非平面结构。关于中心金属和配位部位之间的夹角值,已经进行并讨论了规则几何形变的程度。该Ë HOMO和Ë LUMO研究的配体及其配合物的能量用于计算整体性质。非线性光学参数(NLO),各向异性的polarizibility(Δ的α),且平均第一阶超极化率(< β >)进行了计算。将(< β >)值与作为参考分子的尿素进行比较,(< β >)值的结果表明配体和所研究的配合物具有良好的NLO行为。


























 京公网安备 11010802027423号
京公网安备 11010802027423号