当前位置:
X-MOL 学术
›
J. Iran. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
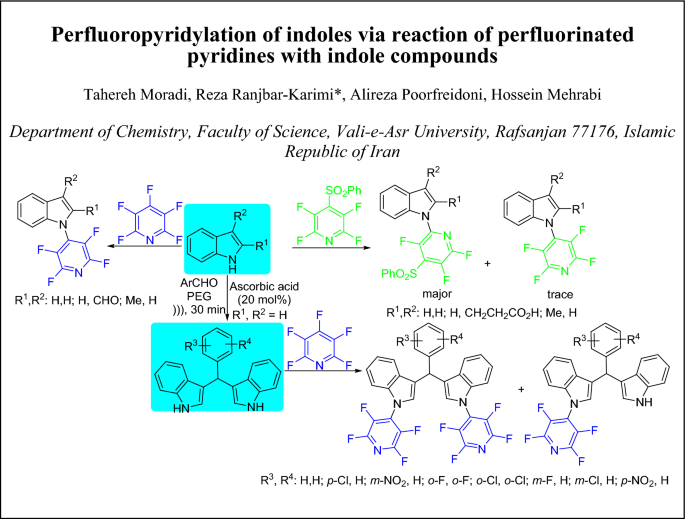
Perfluoropyridylation of indoles via reaction of perfluorinated pyridines with indole compounds
Journal of the Iranian Chemical Society ( IF 2.4 ) Pub Date : 2020-01-27 , DOI: 10.1007/s13738-020-01858-6 Tahereh Moradi , Reza Ranjbar-Karimi , Alireza Poorfreidoni , Hossein Mehrabi
中文翻译:

通过全氟吡啶与吲哚化合物的反应使吲哚全氟吡啶化

Journal of the Iranian Chemical Society ( IF 2.4 ) Pub Date : 2020-01-27 , DOI: 10.1007/s13738-020-01858-6 Tahereh Moradi , Reza Ranjbar-Karimi , Alireza Poorfreidoni , Hossein Mehrabi
Abstract
The reaction of perfluoropyridines with indoles and bis(indolyl)methanes (BIMs) was investigated. The aromatic nucleophilic substitution of pentafluoropyridine with indoles occurs at the 4-position of pyridine ring by nitrogen site of indolyl anion, while reaction with 2,3,5,6-tetrafluoro-4-(phenylsulfonyl)pyridine gave a mixture of products arising substitution at 2-position of pyridine ring and replacement of phenylsulfonyl group. Furthermore, the reaction of pentafluoropyridine with BIMs yielded mixture of mono- and bis-perfluoropyridyl products.Graphic abstract

中文翻译:

通过全氟吡啶与吲哚化合物的反应使吲哚全氟吡啶化
摘要
研究了全氟吡啶与吲哚和双(吲哚基)甲烷(BIM)的反应。五氟吡啶被吲哚的芳族亲核取代发生在吡啶环的4位上,吲哚阴离子的氮原子位置,而与2,3,5,6-四氟-4-(苯磺酰基)吡啶的反应则产生了取代产物在吡啶环的2-位上取代苯基磺酰基。此外,五氟吡啶与BIM的反应产生了单-和双-全氟吡啶基产物的混合物。图形摘要




























 京公网安备 11010802027423号
京公网安备 11010802027423号