当前位置:
X-MOL 学术
›
Enzyme Microb. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The hydrolysis of indoxyl acetate: a versatile reaction to assay carbonic anhydrase activity by high-throughput screening
Enzyme and Microbial Technology ( IF 3.4 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.enzmictec.2020.109584 Mantas Baliukynas 1 , Aušra Veteikytė 1 , Visvaldas Kairys 2 , Inga Matijošytė 1
Enzyme and Microbial Technology ( IF 3.4 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.enzmictec.2020.109584 Mantas Baliukynas 1 , Aušra Veteikytė 1 , Visvaldas Kairys 2 , Inga Matijošytė 1
Affiliation
|
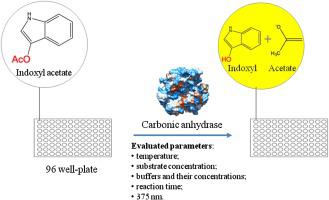
The interest in CO2 capture and conversion by biological methodologies into various beneficial products is increased. In nature, there are enzymes, with hydration activity, which catalyze the reversible hydration of the CO2 molecule and, thus, are of high interest for biotechnological applications. Such enzymes are carbonic anhydrases (CAs). Structural, functional and mutational studies have shown, that besides hydratase activity, CAs have exposed hydrolytic, particularly esterase activity, and importantly, both activities follow similar catalytic mechanisms in the same catalytic pocket. CAs activity measurement methods based on electrometric assays for hydration activity and nitrophenyl based esters for hydrolytic activity assays do not fulfill the requirements amenable for enzyme activity screening methods. By this study, we were aiming to develop an indigogenic assay method based on the esterase activity of CAs. The first time use of indoxyl acetate as a substrate for CA has shown promising results to gain simplicity, repeatability, and applicability to implement high-throughput screening methods.
中文翻译:

醋酸吲哚酚的水解:通过高通量筛选测定碳酸酐酶活性的通用反应
对通过生物方法捕获 CO2 并将其转化为各种有益产品的兴趣正在增加。在自然界中,存在具有水合活性的酶,可催化 CO2 分子的可逆水合,因此对生物技术应用具有很高的兴趣。这种酶是碳酸酐酶(CA)。结构、功能和突变研究表明,除了水合酶活性外,CA 还具有水解,特别是酯酶活性,重要的是,这两种活性在同一催化口袋中遵循相似的催化机制。基于用于水合活性的电测法和用于水解活性的硝基苯基酯的 CA 活性测量方法不满足适用于酶活性筛选方法的要求。通过这项研究,我们的目标是开发一种基于 CA 酯酶活性的靛蓝检测方法。首次使用醋酸吲哚酚作为 CA 的底物已显示出有希望的结果,以实现高通量筛选方法的简单性、可重复性和适用性。
更新日期:2020-09-01
中文翻译:

醋酸吲哚酚的水解:通过高通量筛选测定碳酸酐酶活性的通用反应
对通过生物方法捕获 CO2 并将其转化为各种有益产品的兴趣正在增加。在自然界中,存在具有水合活性的酶,可催化 CO2 分子的可逆水合,因此对生物技术应用具有很高的兴趣。这种酶是碳酸酐酶(CA)。结构、功能和突变研究表明,除了水合酶活性外,CA 还具有水解,特别是酯酶活性,重要的是,这两种活性在同一催化口袋中遵循相似的催化机制。基于用于水合活性的电测法和用于水解活性的硝基苯基酯的 CA 活性测量方法不满足适用于酶活性筛选方法的要求。通过这项研究,我们的目标是开发一种基于 CA 酯酶活性的靛蓝检测方法。首次使用醋酸吲哚酚作为 CA 的底物已显示出有希望的结果,以实现高通量筛选方法的简单性、可重复性和适用性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号