当前位置:
X-MOL 学术
›
Neurosci. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Proteomic analysis involved with synaptic plasticity improvement by GABAA receptor blockade in hippocampus of a mouse model of Alzheimer’s disease
Neuroscience Research ( IF 2.9 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.neures.2020.04.004 Keiichi Kadoyama 1 , Kenji Matsuura 2 , Masaoki Takano 3 , Mieko Otani 3 , Takami Tomiyama 4 , Hiroshi Mori 5 , Shogo Matsuyama 6
Neuroscience Research ( IF 2.9 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.neures.2020.04.004 Keiichi Kadoyama 1 , Kenji Matsuura 2 , Masaoki Takano 3 , Mieko Otani 3 , Takami Tomiyama 4 , Hiroshi Mori 5 , Shogo Matsuyama 6
Affiliation
|
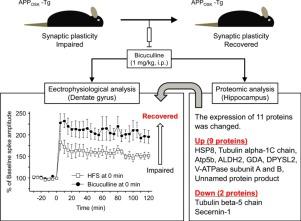
GABAergic system plays a part in synaptic plasticity in the hippocampus. We had reported a long-term potentiation (LTP)-like facilitation in vivo, known as synaptic plasticity, through GABAA receptor blockade by bicuculline and the expression of proteins involved with this synaptic plasticity in mouse hippocampus. In the present study, we aimed to show improvement of impaired synaptic plasticity through GABAA receptor blockade and to clarify the molecular mechanisms involved with this improvement in the hippocampus of mice overexpressing human amyloid precursor protein with the E693Δ mutation (APPOSK-Tg) as an Alzheimer's disease model showing impaired synaptic plasticity. Electrophysiological study showed that the LTP-like facilitation expressed with application of bicuculline in vivo was significantly greater than impaired tetanic LTP in APPOSK-Tg mice, which was improved by bicuculline. Proteomic analysis showed that the expression of 11 proteins in the hippocampus was significantly changed 8 h after bicuculline application to APPOSK-Tg mice. The identified proteins could be functionally classified as chaperome, cytoskeletal protein, energy metabolism, metabolism, neuronal development, and synaptic component. Additionally, western blotting validated the changes in four proteins. We therefore propose that the improvement of impaired synaptic plasticity through GABAA receptor blockade could be mediated by the changed expression of these proteins.
中文翻译:

阿尔茨海默病小鼠模型海马区 GABAA 受体阻断与突触可塑性改善相关的蛋白质组学分析
GABAergic 系统在海马体的突触可塑性中起作用。我们已经报道了体内长时程增强 (LTP) 样的促进作用,称为突触可塑性,通过荷包牡丹碱对 GABAA 受体的阻断以及与小鼠海马中这种突触可塑性有关的蛋白质的表达。在本研究中,我们旨在通过 GABAA 受体阻断来改善受损的突触可塑性,并阐明与这种改善的分子机制有关,这种改善在过表达具有 E693Δ 突变的人类淀粉样前体蛋白(APPOSK-Tg)作为阿尔茨海默病的小鼠的海马中疾病模型显示突触可塑性受损。电生理研究表明,在 APOSK-Tg 小鼠体内,应用荷包牡丹碱表达的 LTP 样促进作用显着大于受损的强直性 LTP,而荷包牡丹碱可以改善这种促进作用。蛋白质组学分析表明,荷包牡丹碱应用于 APOSK-Tg 小鼠后 8 小时,海马区 11 种蛋白质的表达发生了显着变化。鉴定出的蛋白质在功能上可分为分子伴侣、细胞骨架蛋白、能量代谢、代谢、神经元发育和突触成分。此外,蛋白质印迹验证了四种蛋白质的变化。因此,我们提出通过 GABAA 受体阻断改善受损突触可塑性可能是由这些蛋白质的表达改变介导的。蛋白质组学分析表明,荷包牡丹碱应用于 APOSK-Tg 小鼠后 8 小时,海马区 11 种蛋白质的表达发生了显着变化。鉴定出的蛋白质在功能上可分为分子伴侣、细胞骨架蛋白、能量代谢、代谢、神经元发育和突触成分。此外,蛋白质印迹验证了四种蛋白质的变化。因此,我们提出通过 GABAA 受体阻断改善受损突触可塑性可能是由这些蛋白质的表达改变介导的。蛋白质组学分析表明,荷包牡丹碱应用于 APOSK-Tg 小鼠后 8 小时,海马区 11 种蛋白质的表达发生了显着变化。鉴定出的蛋白质在功能上可分为分子伴侣、细胞骨架蛋白、能量代谢、代谢、神经元发育和突触成分。此外,蛋白质印迹验证了四种蛋白质的变化。因此,我们提出通过 GABAA 受体阻断改善受损突触可塑性可能是由这些蛋白质的表达改变介导的。和突触成分。此外,蛋白质印迹验证了四种蛋白质的变化。因此,我们提出通过 GABAA 受体阻断改善受损突触可塑性可能是由这些蛋白质的表达改变介导的。和突触成分。此外,蛋白质印迹验证了四种蛋白质的变化。因此,我们提出通过 GABAA 受体阻断改善受损突触可塑性可能是由这些蛋白质的表达改变介导的。
更新日期:2020-04-01
中文翻译:

阿尔茨海默病小鼠模型海马区 GABAA 受体阻断与突触可塑性改善相关的蛋白质组学分析
GABAergic 系统在海马体的突触可塑性中起作用。我们已经报道了体内长时程增强 (LTP) 样的促进作用,称为突触可塑性,通过荷包牡丹碱对 GABAA 受体的阻断以及与小鼠海马中这种突触可塑性有关的蛋白质的表达。在本研究中,我们旨在通过 GABAA 受体阻断来改善受损的突触可塑性,并阐明与这种改善的分子机制有关,这种改善在过表达具有 E693Δ 突变的人类淀粉样前体蛋白(APPOSK-Tg)作为阿尔茨海默病的小鼠的海马中疾病模型显示突触可塑性受损。电生理研究表明,在 APOSK-Tg 小鼠体内,应用荷包牡丹碱表达的 LTP 样促进作用显着大于受损的强直性 LTP,而荷包牡丹碱可以改善这种促进作用。蛋白质组学分析表明,荷包牡丹碱应用于 APOSK-Tg 小鼠后 8 小时,海马区 11 种蛋白质的表达发生了显着变化。鉴定出的蛋白质在功能上可分为分子伴侣、细胞骨架蛋白、能量代谢、代谢、神经元发育和突触成分。此外,蛋白质印迹验证了四种蛋白质的变化。因此,我们提出通过 GABAA 受体阻断改善受损突触可塑性可能是由这些蛋白质的表达改变介导的。蛋白质组学分析表明,荷包牡丹碱应用于 APOSK-Tg 小鼠后 8 小时,海马区 11 种蛋白质的表达发生了显着变化。鉴定出的蛋白质在功能上可分为分子伴侣、细胞骨架蛋白、能量代谢、代谢、神经元发育和突触成分。此外,蛋白质印迹验证了四种蛋白质的变化。因此,我们提出通过 GABAA 受体阻断改善受损突触可塑性可能是由这些蛋白质的表达改变介导的。蛋白质组学分析表明,荷包牡丹碱应用于 APOSK-Tg 小鼠后 8 小时,海马区 11 种蛋白质的表达发生了显着变化。鉴定出的蛋白质在功能上可分为分子伴侣、细胞骨架蛋白、能量代谢、代谢、神经元发育和突触成分。此外,蛋白质印迹验证了四种蛋白质的变化。因此,我们提出通过 GABAA 受体阻断改善受损突触可塑性可能是由这些蛋白质的表达改变介导的。和突触成分。此外,蛋白质印迹验证了四种蛋白质的变化。因此,我们提出通过 GABAA 受体阻断改善受损突触可塑性可能是由这些蛋白质的表达改变介导的。和突触成分。此外,蛋白质印迹验证了四种蛋白质的变化。因此,我们提出通过 GABAA 受体阻断改善受损突触可塑性可能是由这些蛋白质的表达改变介导的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号