当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Most Influential Physicochemical and In Vitro Assay Descriptors for Hepatotoxicity and Nephrotoxicity Prediction.
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2020-04-27 , DOI: 10.1021/acs.chemrestox.0c00040 Payal Rana 1 , Stephen Kogut 2 , Xuerong Wen 2 , Fatemeh Akhlaghi 2 , Michael D Aleo 1
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2020-04-27 , DOI: 10.1021/acs.chemrestox.0c00040 Payal Rana 1 , Stephen Kogut 2 , Xuerong Wen 2 , Fatemeh Akhlaghi 2 , Michael D Aleo 1
Affiliation
|
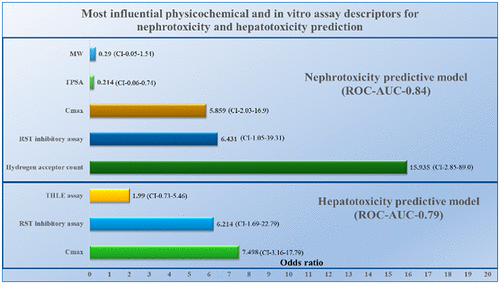
Drug-induced organ injury is a major reason for drug candidate attrition in preclinical and clinical drug development. The liver, kidneys, and heart have been recognized as the most common organ systems affected in safety-related attrition or the subject of black box warnings and postmarket drug withdrawals. In silico physicochemical property calculations and in vitro assays have been utilized separately in the early stages of the drug discovery and development process to predict drug safety. In this study, we combined physicochemical properties and in vitro cytotoxicity assays including mitochondrial dysfunction to build organ-specific univariate and multivariable logistic regression models to achieve odds ratios for the prediction of clinical hepatotoxicity, nephrotoxicity, and cardiotoxicity using 215 marketed drugs. The multivariable hepatotoxic predictive model showed an odds ratio of 6.2 (95% confidence interval (CI) 1.7–22.8) or 7.5 (95% CI 3.2–17.8) for mitochondrial inhibition or drug plasma Cmax >1 μM for drugs associated with liver injury, respectively. The multivariable nephrotoxicity predictive model showed an odds ratio of 5.8 (95% CI 2.0–16.9), 6.4 (95% CI 1.1–39.3), or 15.9 (95% CI 2.8–89.0) for drug plasma Cmax >1 μM, mitochondrial inhibition, or hydrogen-bond-acceptor atoms >7 for drugs associated with kidney injury, respectively. Conversely, drugs with a total polar surface area ≥75 Å were 79% (odds ratio 0.21, 95% CI 0.061–0.74) less likely to be associated with kidney injury. Drugs belonging to the extended clearance classification system (ECCS) class 4, where renal secretion is the primary clearance mechanism (low permeability drugs that are bases/neutrals), were 4 (95% CI 1.8–9.5) times more likely to to be associated with kidney injury with this data set. Alternatively, ECCS class 2 drugs, where hepatic metabolism is the primary clearance (high permeability drugs that are bases/neutrals) were 77% less likely (odds ratio 0.23 95% CI 0.095–0.54) to to be associated with kidney injury. A cardiotoxicity model was poorly defined using any of these drug physicochemical attributes. Combining in silico physicochemical properties descriptors along with in vitro toxicity assays can be used to build predictive toxicity models to select small molecule therapeutics with less potential to cause liver and kidney organ toxicity.
中文翻译:

肝毒性和肾毒性预测的最具影响力的理化和体外测定指标。
药物诱发的器官损伤是临床前和临床药物开发中候选药物耗竭的主要原因。肝脏,肾脏和心脏已被公认为是受到与安全有关的损耗或黑匣子警告和上市后停药影响的最常见器官系统。在药物发现和开发过程的早期阶段,已分别利用计算机化学物理化学性质计算和体外测定来预测药物安全性。在这项研究中,我们结合了理化特性和体外细胞毒性测定法(包括线粒体功能障碍),建立了器官特异性单变量和多变量logistic回归模型,以使用215种市售药物获得的临床肝毒性,肾毒性和心脏毒性预测的比值比。与肝损伤相关的药物的C max > 1μM。多变量肾毒性预测模型显示,血浆C max的比值比为5.8(95%CI 2.0-16.9),6.4(95%CI 1.1-39.3)或15.9(95%CI 2.8-89.0)> 1μM,线粒体抑制或氢键受体原子> 7分别与肾脏损伤相关。相反,总极性表面积≥75Å的药物与肾脏损伤相关的可能性降低了79%(几率0.21,95%CI 0.061-0.74)。属于扩展清除分类系统(ECCS)4类的药物(其中以肾分泌为主要清除机制(低渗透性药物为基础/中性))发生关联的可能性高4倍(95%CI 1.8–9.5)该数据集对肾脏损伤有帮助。另外,以ECCS 2类药物为主要清除对象的是肝代谢(基础/中性的高渗透性药物),其与肾脏损伤相关的可能性降低了77%(几率0.23 95%CI 0.095-0.54)。使用这些药物的任何物理化学属性,对心脏毒性模型的定义都较差。结合计算机理化特性描述符和体外毒性测定可用于建立预测毒性模型,以选择可能引起肝和肾器官毒性的可能性较小的小分子疗法。
更新日期:2020-04-27
中文翻译:

肝毒性和肾毒性预测的最具影响力的理化和体外测定指标。
药物诱发的器官损伤是临床前和临床药物开发中候选药物耗竭的主要原因。肝脏,肾脏和心脏已被公认为是受到与安全有关的损耗或黑匣子警告和上市后停药影响的最常见器官系统。在药物发现和开发过程的早期阶段,已分别利用计算机化学物理化学性质计算和体外测定来预测药物安全性。在这项研究中,我们结合了理化特性和体外细胞毒性测定法(包括线粒体功能障碍),建立了器官特异性单变量和多变量logistic回归模型,以使用215种市售药物获得的临床肝毒性,肾毒性和心脏毒性预测的比值比。与肝损伤相关的药物的C max > 1μM。多变量肾毒性预测模型显示,血浆C max的比值比为5.8(95%CI 2.0-16.9),6.4(95%CI 1.1-39.3)或15.9(95%CI 2.8-89.0)> 1μM,线粒体抑制或氢键受体原子> 7分别与肾脏损伤相关。相反,总极性表面积≥75Å的药物与肾脏损伤相关的可能性降低了79%(几率0.21,95%CI 0.061-0.74)。属于扩展清除分类系统(ECCS)4类的药物(其中以肾分泌为主要清除机制(低渗透性药物为基础/中性))发生关联的可能性高4倍(95%CI 1.8–9.5)该数据集对肾脏损伤有帮助。另外,以ECCS 2类药物为主要清除对象的是肝代谢(基础/中性的高渗透性药物),其与肾脏损伤相关的可能性降低了77%(几率0.23 95%CI 0.095-0.54)。使用这些药物的任何物理化学属性,对心脏毒性模型的定义都较差。结合计算机理化特性描述符和体外毒性测定可用于建立预测毒性模型,以选择可能引起肝和肾器官毒性的可能性较小的小分子疗法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号