当前位置:
X-MOL 学术
›
J. Mol. Cell. Cardiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A distinct molecular mechanism by which phenytoin rescues a novel long QT 3 variant.
Journal of Molecular and Cellular Cardiology ( IF 5 ) Pub Date : 2020-04-24 , DOI: 10.1016/j.yjmcc.2020.04.027 Ivan Gando 1 , Chiara Campana 2 , Reina Bianca Tan 1 , Frank Cecchin 1 , Eric A Sobie 2 , William A Coetzee 3
Journal of Molecular and Cellular Cardiology ( IF 5 ) Pub Date : 2020-04-24 , DOI: 10.1016/j.yjmcc.2020.04.027 Ivan Gando 1 , Chiara Campana 2 , Reina Bianca Tan 1 , Frank Cecchin 1 , Eric A Sobie 2 , William A Coetzee 3
Affiliation
|
BACKGROUND
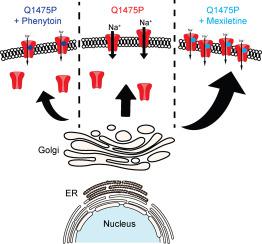
Genetic variants in SCN5A can result in channelopathies such as the long QT syndrome type 3 (LQT3), but the therapeutic response to Na+ channel blockers can vary. We previously reported a case of an infant with malignant LQT3 and a missense Q1475P SCN5A variant, who was effectively treated with phenytoin, but only partially with mexiletine. Here, we functionally characterized this variant and investigated possible mechanisms for the differential drug actions.
METHODS
Wild-type or mutant Nav1.5 cDNAs were examined in transfected HEK293 cells with patch clamping and biochemical assays. We used computational modeling to provide insights into altered channel kinetics and to predict effects on the action potential.
RESULTS
The Q1475P variant in Nav1.5 reduced the current density and channel surface expression, characteristic of a trafficking defect. The variant also led to positive shifts in the voltage dependence of steady-state activation and inactivation, faster inactivation and recovery from inactivation, and increased the "late" Na+ current. Simulations of Nav1.5 gating with a 9-state Markov model suggested that transitions from inactivated to closed states were accelerated in Q1475P channels, leading to accumulation of channels in non-inactivated closed states. Simulations with a human ventricular myocyte model predicted action potential prolongation with Q1475P, compared with wild type, channels. Patch clamp data showed that mexiletine and phenytoin similarly rescued some of the gating defects. Chronic incubation with mexiletine, but not phenytoin, rescued the Nav1.5-Q1475P trafficking defect, thus increasing mutant channel expression.
CONCLUSIONS
The gain-of-function effects of Nav1.5-Q1475P predominate to cause a malignant long QT phenotype. Phenytoin partially corrects the gating defect without restoring surface expression of the mutant channel, whereas mexiletine restores surface expression of the mutant channel, which may explain the lack of efficacy of mexiletine when compared to phenytoin. Our data makes a case for experimental studies before embarking on a one-for-all therapy of arrhythmias.
中文翻译:

苯妥英钠拯救新的长QT 3变体的独特分子机制。
背景技术SCN5A中的遗传变异可导致通道病,例如长QT综合征3型(LQT3),但对Na +通道阻滞剂的治疗反应可能会有所不同。我们先前曾报道一例患恶性LQT3和错义Q1475P SCN5A变异的婴儿的病例,该患者接受苯妥英钠有效治疗,但仅部分接受美西律治疗。在这里,我们在功能上表征了该变体,并研究了不同药物作用的可能机制。方法在野生型或突变的Nav1.5 cDNAs中,通过膜片钳和生物化学检测转染的HEK293细胞。我们使用计算模型来洞察改变的通道动力学并预测对动作电位的影响。结果Nav1.5中的Q1475P变体降低了电流密度和通道表面表达,交易缺陷的特征。该变体还导致稳态激活和失活的电压依赖性正向变化,更快的失活和从失活中恢复,并增加了“晚期” Na +电流。用9状态马尔可夫模型对Nav1.5门控进行的仿真表明,Q1475P通道中从非活动状态到闭合状态的过渡被加速,从而导致非活动状态下的通道积累。与野生型通道相比,使用人类心室肌细胞模型进行的模拟预测了Q1475P的动作电位延长。膜片钳数据显示美西律和苯妥英钠同样可以挽救一些门控缺陷。与美西律的长期温育,而非苯妥英的温育,挽救了Nav1.5-Q1475P转运缺陷,从而增加了突变体通道的表达。结论Nav1.5-Q1475P的功能获得作用主要导致长QT恶性表型。苯妥英钠可部分纠正门控缺陷而不恢复突变体通道的表面表达,而美西律可恢复突变体通道的表面表达,这可能解释了与苯妥英钠相比美西律缺乏疗效。我们的数据为进行心律失常的全面治疗提供了实验研究依据。
更新日期:2020-04-24
中文翻译:

苯妥英钠拯救新的长QT 3变体的独特分子机制。
背景技术SCN5A中的遗传变异可导致通道病,例如长QT综合征3型(LQT3),但对Na +通道阻滞剂的治疗反应可能会有所不同。我们先前曾报道一例患恶性LQT3和错义Q1475P SCN5A变异的婴儿的病例,该患者接受苯妥英钠有效治疗,但仅部分接受美西律治疗。在这里,我们在功能上表征了该变体,并研究了不同药物作用的可能机制。方法在野生型或突变的Nav1.5 cDNAs中,通过膜片钳和生物化学检测转染的HEK293细胞。我们使用计算模型来洞察改变的通道动力学并预测对动作电位的影响。结果Nav1.5中的Q1475P变体降低了电流密度和通道表面表达,交易缺陷的特征。该变体还导致稳态激活和失活的电压依赖性正向变化,更快的失活和从失活中恢复,并增加了“晚期” Na +电流。用9状态马尔可夫模型对Nav1.5门控进行的仿真表明,Q1475P通道中从非活动状态到闭合状态的过渡被加速,从而导致非活动状态下的通道积累。与野生型通道相比,使用人类心室肌细胞模型进行的模拟预测了Q1475P的动作电位延长。膜片钳数据显示美西律和苯妥英钠同样可以挽救一些门控缺陷。与美西律的长期温育,而非苯妥英的温育,挽救了Nav1.5-Q1475P转运缺陷,从而增加了突变体通道的表达。结论Nav1.5-Q1475P的功能获得作用主要导致长QT恶性表型。苯妥英钠可部分纠正门控缺陷而不恢复突变体通道的表面表达,而美西律可恢复突变体通道的表面表达,这可能解释了与苯妥英钠相比美西律缺乏疗效。我们的数据为进行心律失常的全面治疗提供了实验研究依据。


























 京公网安备 11010802027423号
京公网安备 11010802027423号