当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Covalently Immobilizing Interferon-γ Drives Filopodia Production through Specific Receptor-Ligand Interactions Independently of Canonical Downstream Signaling.
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2020-05-11 , DOI: 10.1021/acs.bioconjchem.0c00105 Shaun M Christie 1 , Trevor R Ham 2 , Grant T Gilmore 1 , Paul D Toth 1 , Nic D Leipzig 2, 3 , Adam W Smith 1
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2020-05-11 , DOI: 10.1021/acs.bioconjchem.0c00105 Shaun M Christie 1 , Trevor R Ham 2 , Grant T Gilmore 1 , Paul D Toth 1 , Nic D Leipzig 2, 3 , Adam W Smith 1
Affiliation
|
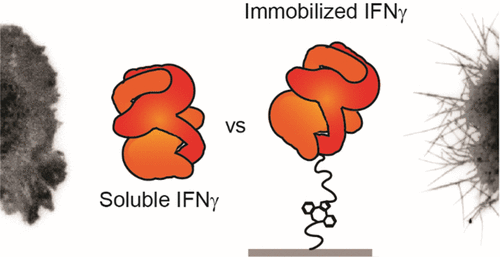
Immobilizing a signaling protein to guide cell behavior has been employed in a wide variety of studies. This approach draws inspiration from biology, where specific, affinity-based interactions between membrane receptors and immobilized proteins in the extracellular matrix guide many developmental and homeostatic processes. Synthetic immobilization approaches, however, do not necessarily recapitulate the in vivo signaling system and potentially lead to artificial receptor-ligand interactions. To investigate the effects of one example of engineered receptor-ligand interactions, we focus on the immobilization of interferon-γ (IFN-γ), which has been used to drive differentiation of neural stem cells (NSCs). To isolate the effect of ligand immobilization, we transfected Cos-7 cells with only interferon-γ receptor 1 (IFNγR1), not IFNγR2, so that the cells could bind IFN-γ but were incapable of canonical signal transduction. We then exposed the cells to surfaces containing covalently immobilized IFN-γ and studied membrane morphology, receptor-ligand dynamics, and receptor activation. We found that exposing cells to immobilized but not soluble IFN-γ drove the formation of filopodia in both NSCs and Cos-7, showing that covalently immobilizing IFN-γ is enough to affect cell behavior, independently of canonical downstream signaling. Overall, this work suggests that synthetic growth factor immobilization can influence cell morphology beyond enhancing canonical cell responses through the prolonged signaling duration or spatial patterning enabled by protein immobilization. This suggests that differentiation of NSCs could be driven by canonical and non-canonical pathways when IFN-γ is covalently immobilized. This finding has broad implications for bioengineering approaches to guide cell behavior, as one ligand has the potential to impact multiple pathways even when cells lack the canonical signal transduction machinery.
中文翻译:

共价固定干扰素-γ 通过独立于典型下游信号的特定受体-配体相互作用驱动丝状伪足的产生。
固定信号蛋白以指导细胞行为已被广泛用于各种研究中。这种方法从生物学中汲取灵感,其中膜受体与细胞外基质中固定化蛋白质之间基于亲和力的特异性相互作用指导许多发育和稳态过程。然而,合成固定方法不一定重述体内信号系统,并可能导致人工受体-配体相互作用。为了研究工程化受体-配体相互作用的一个例子的影响,我们专注于干扰素-γ (IFN-γ) 的固定化,它已被用于驱动神经干细胞 (NSC) 的分化。为了隔离配体固定化的影响,我们仅用干扰素-γ受体 1 (IFNγR1) 转染 Cos-7 细胞,而不用 IFNγR2,因此细胞可以结合 IFN-γ 但不能进行典型的信号转导。然后,我们将细胞暴露于含有共价固定化 IFN-γ 的表面,并研究了膜形态、受体-配体动力学和受体激活。我们发现将细胞暴露于固定化但不可溶的 IFN-γ 会促进 NSC 和 Cos-7 中丝状伪足的形成,这表明共价固定化 IFN-γ 足以影响细胞行为,而与典型的下游信号传导无关。总的来说,这项工作表明,合成生长因子固定可以影响细胞形态,而不仅仅是通过延长信号持续时间或蛋白质固定启用的空间模式来增强典型细胞反应。这表明当 IFN-γ 被共价固定时,NSC 的分化可能由经典和非经典途径驱动。这一发现对于指导细胞行为的生物工程方法具有广泛的意义,因为即使细胞缺乏规范的信号转导机制,一个配体也有可能影响多种途径。
更新日期:2020-04-24
中文翻译:

共价固定干扰素-γ 通过独立于典型下游信号的特定受体-配体相互作用驱动丝状伪足的产生。
固定信号蛋白以指导细胞行为已被广泛用于各种研究中。这种方法从生物学中汲取灵感,其中膜受体与细胞外基质中固定化蛋白质之间基于亲和力的特异性相互作用指导许多发育和稳态过程。然而,合成固定方法不一定重述体内信号系统,并可能导致人工受体-配体相互作用。为了研究工程化受体-配体相互作用的一个例子的影响,我们专注于干扰素-γ (IFN-γ) 的固定化,它已被用于驱动神经干细胞 (NSC) 的分化。为了隔离配体固定化的影响,我们仅用干扰素-γ受体 1 (IFNγR1) 转染 Cos-7 细胞,而不用 IFNγR2,因此细胞可以结合 IFN-γ 但不能进行典型的信号转导。然后,我们将细胞暴露于含有共价固定化 IFN-γ 的表面,并研究了膜形态、受体-配体动力学和受体激活。我们发现将细胞暴露于固定化但不可溶的 IFN-γ 会促进 NSC 和 Cos-7 中丝状伪足的形成,这表明共价固定化 IFN-γ 足以影响细胞行为,而与典型的下游信号传导无关。总的来说,这项工作表明,合成生长因子固定可以影响细胞形态,而不仅仅是通过延长信号持续时间或蛋白质固定启用的空间模式来增强典型细胞反应。这表明当 IFN-γ 被共价固定时,NSC 的分化可能由经典和非经典途径驱动。这一发现对于指导细胞行为的生物工程方法具有广泛的意义,因为即使细胞缺乏规范的信号转导机制,一个配体也有可能影响多种途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号