当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Bacteriophage Inspired Growth-Decoupled Recombinant Protein Production in Escherichia coli.
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2020-04-23 , DOI: 10.1021/acssynbio.0c00028 Patrick Stargardt 1 , Lukas Feuchtenhofer 1 , Monika Cserjan-Puschmann 2 , Gerald Striedner 2 , Juergen Mairhofer 1
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2020-04-23 , DOI: 10.1021/acssynbio.0c00028 Patrick Stargardt 1 , Lukas Feuchtenhofer 1 , Monika Cserjan-Puschmann 2 , Gerald Striedner 2 , Juergen Mairhofer 1
Affiliation
|
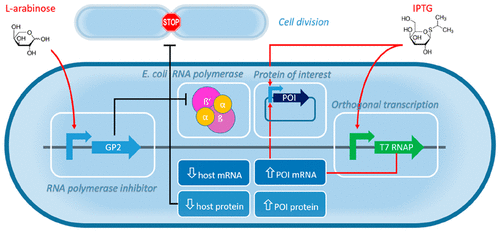
Modulating resource allocation in bacteria to redirect metabolic building blocks to the formation of recombinant proteins rather than biomass formation remains a grand challenge in biotechnology. Here, we present a novel approach for improved recombinant protein production (RPP) using Escherichia coli (E. coli) by decoupling recombinant protein synthesis from cell growth. We show that cell division and host mRNA transcription can be successfully inhibited by coexpression of a bacteriophage-derived E. coli RNA polymerase (RNAP) inhibitor peptide and that genes overtranscribed by the orthogonal T7 RNAP can finally account to >55% of cell dry mass (CDM). This RNAP inhibitor peptide binds the E. coli RNAP and therefore prevents σ-factor 70 mediated formation of transcriptional qualified open promoter complexes. Thereby, the transcription of σ-factor 70 driven host genes is inhibited, and metabolic resources can be exclusively utilized for synthesis of the protein of interest (POI). Here, we mimic the late phase of bacteriophage infection by coexpressing a phage-derived xenogeneic regulator that reprograms the host cell and thereby are able to significantly improve RPP under industrial relevant fed-batch process conditions at bioreactor scale. We have evaluated production of several different recombinant proteins at different scales (from microscale to 20 L fed-batch scale) and have been able to improve total and soluble proteins yields up to 3.4-fold in comparison to the reference expression system E. coli BL21(DE3). This novel approach for growth-decoupled RPP has profound implications for biotechnology and bioengineering and helps to establish more cost-effective and generic manufacturing processes for biologics and biomaterials.
中文翻译:

噬菌体启发大肠杆菌中的生长解耦重组蛋白生产。
调节细菌中的资源分配以将代谢构件重新定向到重组蛋白的形成而不是生物质的形成仍然是生物技术领域的巨大挑战。在这里,我们提出了一种通过将重组蛋白合成与细胞生长脱钩的方法,利用大肠杆菌(E. coli)改善重组蛋白生产(RPP)的新方法。我们表明,可以通过共表达噬菌体来源的大肠杆菌RNA聚合酶(RNAP)抑制剂肽成功抑制细胞分裂和宿主mRNA转录,并且正交T7 RNAP过度转录的基因最终可以占细胞干重的> 55% (CDM)。该RNAP抑制剂肽结合大肠杆菌RNAP并因此阻止了σ因子70介导的转录合格开放启动子复合物的形成。由此,抑制了由σ-因子70驱动的宿主基因的转录,并且代谢资源可以专门用于合成目的蛋白质(POI)。在这里,我们通过共表达可重新编程宿主细胞的噬菌体衍生的异种调节剂来模拟噬菌体感染的后期阶段,从而能够在生物反应器规模的工业相关补料分批工艺条件下显着提高RPP。我们已经评估了几种不同规模(从微型规模到20 L分批补料规模)的不同重组蛋白的生产,与参考表达系统大肠杆菌相比,我们能够将总蛋白和可溶性蛋白的产量提高多达3.4倍。BL21(DE3)。这种用于增长解耦的RPP的新颖方法对生物技术和生物工程学具有深远的意义,并有助于为生物制品和生物材料建立更具成本效益的通用制造工艺。
更新日期:2020-06-19
中文翻译:

噬菌体启发大肠杆菌中的生长解耦重组蛋白生产。
调节细菌中的资源分配以将代谢构件重新定向到重组蛋白的形成而不是生物质的形成仍然是生物技术领域的巨大挑战。在这里,我们提出了一种通过将重组蛋白合成与细胞生长脱钩的方法,利用大肠杆菌(E. coli)改善重组蛋白生产(RPP)的新方法。我们表明,可以通过共表达噬菌体来源的大肠杆菌RNA聚合酶(RNAP)抑制剂肽成功抑制细胞分裂和宿主mRNA转录,并且正交T7 RNAP过度转录的基因最终可以占细胞干重的> 55% (CDM)。该RNAP抑制剂肽结合大肠杆菌RNAP并因此阻止了σ因子70介导的转录合格开放启动子复合物的形成。由此,抑制了由σ-因子70驱动的宿主基因的转录,并且代谢资源可以专门用于合成目的蛋白质(POI)。在这里,我们通过共表达可重新编程宿主细胞的噬菌体衍生的异种调节剂来模拟噬菌体感染的后期阶段,从而能够在生物反应器规模的工业相关补料分批工艺条件下显着提高RPP。我们已经评估了几种不同规模(从微型规模到20 L分批补料规模)的不同重组蛋白的生产,与参考表达系统大肠杆菌相比,我们能够将总蛋白和可溶性蛋白的产量提高多达3.4倍。BL21(DE3)。这种用于增长解耦的RPP的新颖方法对生物技术和生物工程学具有深远的意义,并有助于为生物制品和生物材料建立更具成本效益的通用制造工艺。


























 京公网安备 11010802027423号
京公网安备 11010802027423号