当前位置:
X-MOL 学术
›
Int. J. Mass Spectrom.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fragmentation pathways of odd- and even-electron N-alkylated synthetic cathinones
International Journal of Mass Spectrometry ( IF 1.8 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.ijms.2020.116354 J. Tyler Davidson , Zachary J. Sasiene , Glen P. Jackson
International Journal of Mass Spectrometry ( IF 1.8 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.ijms.2020.116354 J. Tyler Davidson , Zachary J. Sasiene , Glen P. Jackson
|
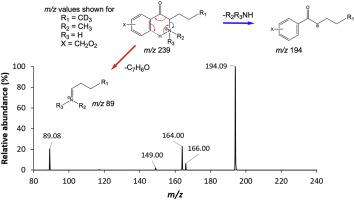
Abstract Three ionization techniques, isotopic labeling, high-resolution mass spectrometry (HRMS) and multi-stage mass spectrometry (MSn) were used to analyze a series of N-alkylated synthetic cathinone derivatives and gain a deeper understanding of their fragmentation behavior during mass spectrometric analysis. The compounds analyzed represent 15 unique structures with common substitutions to the core synthetic cathinone structure, including substitutions to the aromatic ring and the number and types of N-alkyl functionalities. The analytical techniques employed include gas chromatography-electron ionization-mass spectrometry (GC-EI-MS), electrospray ionization-tandem mass spectrometry (ESI-MS/MS) with HRMS and direct analysis in real time tandem mass spectrometry (DART-MS/MS) with HRMS. These techniques cover a variety of forensic applications, including seized drug analysis, toxicological analysis and screening analysis, in local, state and federal laboratories. For collision-induced dissociation (CID) of protonated precursors, the spectra of 2° and 3° amines showed evidence for charge-remote and charge-directed fragmentation mechanisms. The 2° amines lost H2O as a dominant pathway whereas the 3° amines favored the formation of alkylphenones. As reported by others, CID of protonated N-alkylated cathinones containing 2° and 3°amines also provided an abundance of odd-electron product ions from the even-electron precursors. In contrast to the rearrangements observed in CID of protonated cathinones, EI fragmentation patterns were dominated by radical-directed cleavages to form iminium ions and charge-directed cleavages to form acylium ions. A comparison between the fragmentation behaviors of N-alkylated synthetic cathinones under all three ionization techniques enables a deeper understanding of N-alkylated synthetic cathinone fragmentation under varying instrumental setups.
中文翻译:

奇电子和偶电子 N-烷基化合成卡西酮的断裂途径
摘要 采用同位素标记、高分辨质谱 (HRMS) 和多级质谱 (MSn) 三种电离技术对一系列 N-烷基化合成卡西酮衍生物进行了分析,深入了解了它们在质谱分析过程中的碎裂行为。分析。分析的化合物代表了 15 种独特的结构,这些结构具有对核心合成卡西酮结构的共同取代,包括对芳香环的取代以及 N-烷基官能团的数量和类型。所采用的分析技术包括气相色谱-电子电离-质谱 (GC-EI-MS)、电喷雾电离-串联质谱 (ESI-MS/MS) 和 HRMS 和实时串联质谱中的直接分析 (DART-MS/ MS) 与 HRMS。这些技术涵盖了各种取证应用,包括在地方、州和联邦实验室进行的缉获药物分析、毒理学分析和筛选分析。对于质子化前体的碰撞诱导解离 (CID),2° 和 3° 胺的光谱显示了电荷远程和电荷导向断裂机制的证据。2° 胺失去 H2O 作为主要途径,而 3° 胺有利于形成烷基苯酮。正如其他人报道的那样,含有 2° 和 3° 胺的质子化 N-烷基化卡西酮的 CID 也提供了大量来自偶电子前体的奇电子产物离子。与在质子化卡西酮的 CID 中观察到的重排相反,EI 碎裂模式主要由自由基定向裂解形成亚胺离子和电荷定向裂解形成酰基离子。
更新日期:2020-07-01
中文翻译:

奇电子和偶电子 N-烷基化合成卡西酮的断裂途径
摘要 采用同位素标记、高分辨质谱 (HRMS) 和多级质谱 (MSn) 三种电离技术对一系列 N-烷基化合成卡西酮衍生物进行了分析,深入了解了它们在质谱分析过程中的碎裂行为。分析。分析的化合物代表了 15 种独特的结构,这些结构具有对核心合成卡西酮结构的共同取代,包括对芳香环的取代以及 N-烷基官能团的数量和类型。所采用的分析技术包括气相色谱-电子电离-质谱 (GC-EI-MS)、电喷雾电离-串联质谱 (ESI-MS/MS) 和 HRMS 和实时串联质谱中的直接分析 (DART-MS/ MS) 与 HRMS。这些技术涵盖了各种取证应用,包括在地方、州和联邦实验室进行的缉获药物分析、毒理学分析和筛选分析。对于质子化前体的碰撞诱导解离 (CID),2° 和 3° 胺的光谱显示了电荷远程和电荷导向断裂机制的证据。2° 胺失去 H2O 作为主要途径,而 3° 胺有利于形成烷基苯酮。正如其他人报道的那样,含有 2° 和 3° 胺的质子化 N-烷基化卡西酮的 CID 也提供了大量来自偶电子前体的奇电子产物离子。与在质子化卡西酮的 CID 中观察到的重排相反,EI 碎裂模式主要由自由基定向裂解形成亚胺离子和电荷定向裂解形成酰基离子。


























 京公网安备 11010802027423号
京公网安备 11010802027423号