当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalyst-Free Cyclization- and Curtius Rearrangement-Induced Functional Group Transformation: An Improved Synthetic Strategy of First-in-Class ATX Inhibitor Ziritaxestat (GLPG-1690)
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-04-23 , DOI: 10.1021/acs.oprd.9b00511 Hongrui Lei 1 , Yu Yang 1 , Changtao Li 1 , Fang Jia 1 , Nan Jiang 1 , Ping Gong 1 , Xin Zhai 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-04-23 , DOI: 10.1021/acs.oprd.9b00511 Hongrui Lei 1 , Yu Yang 1 , Changtao Li 1 , Fang Jia 1 , Nan Jiang 1 , Ping Gong 1 , Xin Zhai 1
Affiliation
|
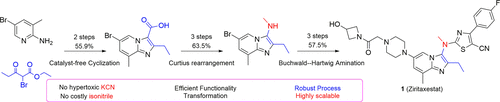
A practical and highly efficient protocol for the production of Autotaxin (ATX) inhibitor Ziritaxestat (1) was described. The procedure began with a catalyst-free bicomponent cyclization for the construction of the imidazo[1,2-a]pyridine skeleton 16. Subsequently, a typical Curtius rearrangement of carboxylic acid 17 followed by nucleophilic attacking of 3,5-dichlorobenzyl alcohol 18f led to the carbamate analogue 19f. N-methylated 20 was readily deprotected through HBr/HOAc treatment, which further conveniently took part in an alternative K2CO3-induced N-alkylation reaction with 9 to give 10. 10 coupled directly with piperazine to furnish 13, which ideally circumvent the removal of the Boc group. As a result, the hypertoxic KCN and the hypertoxic and costly isonitrile 3 involved in the tricomponent cyclization were carefully avoided. Ultimately, a novel, scalable, and cost-effective route was favorably developed to afford Ziritaxestat in a 20.4% overall yield.
中文翻译:

无催化剂的环化和Curtius重排诱导的官能团转化:一流的ATX抑制剂Ziritaxestat(GLPG-1690)的改进合成策略
描述了一种生产Autotaxin(ATX)抑制剂Ziritaxestat(1)的实用且高效的方法。该程序从无催化剂双组分环化开始,用于构建咪唑并[1,2- a ]吡啶骨架16。随后,典型的羧酸17的库尔修斯重排,随后对3,5-二氯苄基醇18f的亲核攻击导致氨基甲酸酯类似物19f。N-甲基化的20很容易通过HBr / HOAc处理脱保护,它进一步方便地参与了另一种K 2 CO 3诱导的N-9的烷基化反应,得到10。10与哌嗪直接偶联,得到13,理想地避免了Boc基团的除去。结果,小心地避免了涉及三组分环化的高毒性KCN和高毒性且昂贵的异腈3。最终,开发出新颖,可扩展且具有成本效益的途径,从而以20.4%的总收率获得了Ziritaxestat。
更新日期:2020-06-19
中文翻译:

无催化剂的环化和Curtius重排诱导的官能团转化:一流的ATX抑制剂Ziritaxestat(GLPG-1690)的改进合成策略
描述了一种生产Autotaxin(ATX)抑制剂Ziritaxestat(1)的实用且高效的方法。该程序从无催化剂双组分环化开始,用于构建咪唑并[1,2- a ]吡啶骨架16。随后,典型的羧酸17的库尔修斯重排,随后对3,5-二氯苄基醇18f的亲核攻击导致氨基甲酸酯类似物19f。N-甲基化的20很容易通过HBr / HOAc处理脱保护,它进一步方便地参与了另一种K 2 CO 3诱导的N-9的烷基化反应,得到10。10与哌嗪直接偶联,得到13,理想地避免了Boc基团的除去。结果,小心地避免了涉及三组分环化的高毒性KCN和高毒性且昂贵的异腈3。最终,开发出新颖,可扩展且具有成本效益的途径,从而以20.4%的总收率获得了Ziritaxestat。


























 京公网安备 11010802027423号
京公网安备 11010802027423号