当前位置:
X-MOL 学术
›
J. Inherit. Metab. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Long-term survival outcomes of patients with Niemann-Pick disease type C receiving miglustat treatment: A large retrospective observational study.
Journal of Inherited Metabolic Disease ( IF 4.2 ) Pub Date : 2020-04-23 , DOI: 10.1002/jimd.12245 Marc C Patterson 1 , William S Garver 2 , Robert Giugliani 3, 4 , Jackie Imrie 5 , Helena Jahnova 6 , F John Meaney 7 , Yann Nadjar 8 , Marie T Vanier 9, 10 , Patrick Moneuse 11 , Olivier Morand 11 , Daniel Rosenberg 12 , Barbara Schwierin 13, 14 , Benedicte Héron 15, 16
Journal of Inherited Metabolic Disease ( IF 4.2 ) Pub Date : 2020-04-23 , DOI: 10.1002/jimd.12245 Marc C Patterson 1 , William S Garver 2 , Robert Giugliani 3, 4 , Jackie Imrie 5 , Helena Jahnova 6 , F John Meaney 7 , Yann Nadjar 8 , Marie T Vanier 9, 10 , Patrick Moneuse 11 , Olivier Morand 11 , Daniel Rosenberg 12 , Barbara Schwierin 13, 14 , Benedicte Héron 15, 16
Affiliation
|
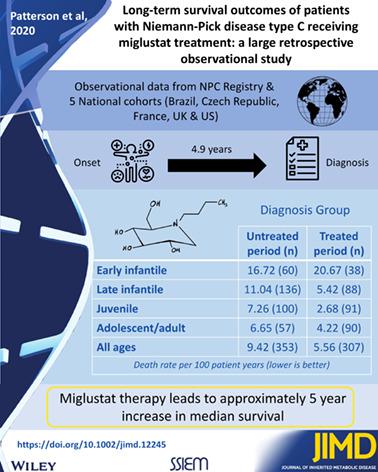
Miglustat has been indicated for the treatment of Niemann‐Pick disease type C (NP‐C) since 2009. The aim of this observational study was to assess the effect of miglustat on long‐term survival of patients with NP‐C. Data for 789 patients from five large national cohorts and from the NPC Registry were collected and combined. Miglustat‐treated and untreated patients overall and within sub‐groups according to age‐at‐neurological‐onset, that is, early infantile‐onset (<2 years), late infantile‐onset (2 to <6 years), juvenile‐onset (6 to <15 years), and adolescent/adult‐onset (≥15 years) were analysed and compared. Survival was analysed from the time of first neurological manifestation (Neurological onset group, comprising 669 patients) and from diagnosis (Diagnosis group, comprising 590 patients) using a Cox proportional hazard model adjusted for various covariates. Overall, 384 (57.4%) patients in the Neurological onset group and 329 (55.8%) in the Diagnosis group were treated with miglustat. Miglustat treatment was associated with a significant reduction in risk of mortality in both groups (entire Neurological onset group, Hazard ratio [HR] = 0.51; entire Diagnosis group, HR = 0.44; both P < .001). The effect was observed consistently in all age‐at‐neurological‐onset sub‐groups (HRs = 0.3 to 0.7) and was statistically significant for late infantile‐onset patients in both groups (Neurological onset group, HR = 0.36, P < .05; Diagnosis group, HR = 0.32, P < .01), and juvenile‐onset patients in the Diagnosis group only (HR = 0.30, P < .05). Despite the limitations of the data that urge cautious interpretation, the findings are consistent with a beneficial effect of miglustat on survival in patients with NP‐C.
中文翻译:

接受米格司他治疗的 C 型尼曼匹克病患者的长期生存结果:一项大型回顾性观察研究。
自 2009 年以来,米格司他已获批用于治疗尼曼匹克病 C 型 (NP-C)。本观察性研究的目的是评估米格司他对 NP-C 患者长期生存的影响。收集并合并了来自五个大型国家队列和 NPC 登记处的 789 名患者的数据。Miglustat 治疗和未治疗的患者总体和亚组内根据神经系统发病年龄,即早期婴儿发病(<2 岁)、晚期婴儿发病(2 至 <6 岁)、青少年发病对(6 至 <15 岁)和青少年/成人发病(≥15 岁)进行了分析和比较。从第一次神经系统表现(神经系统发作组,包括 669 名患者)和诊断(诊断组,包括 590 名患者)使用针对各种协变量调整的 Cox 比例风险模型。总体而言,神经系统发作组的 384 名 (57.4%) 患者和诊断组的 329 名 (55.8%) 患者接受了米格司他治疗。Miglustat 治疗与两组的死亡率风险显着降低相关(整个神经系统发作组,风险比 [HR] = 0.51;整个诊断组,HR = 0.44;两者P <.001)。在所有神经系统发病年龄亚组(HRs = 0.3 至 0.7)中一致观察到这种效果,并且对于两组中晚期婴儿发病患者具有统计学意义(神经系统发病组,HR = 0.36,P < .05 ;诊断组,HR = 0.32,P < .01)和仅诊断组的青少年发病患者(HR = 0.30,P < .05)。尽管数据的局限性促使我们谨慎解释,但研究结果与米格司他对 NP-C 患者生存的有益影响一致。
更新日期:2020-04-23
中文翻译:

接受米格司他治疗的 C 型尼曼匹克病患者的长期生存结果:一项大型回顾性观察研究。
自 2009 年以来,米格司他已获批用于治疗尼曼匹克病 C 型 (NP-C)。本观察性研究的目的是评估米格司他对 NP-C 患者长期生存的影响。收集并合并了来自五个大型国家队列和 NPC 登记处的 789 名患者的数据。Miglustat 治疗和未治疗的患者总体和亚组内根据神经系统发病年龄,即早期婴儿发病(<2 岁)、晚期婴儿发病(2 至 <6 岁)、青少年发病对(6 至 <15 岁)和青少年/成人发病(≥15 岁)进行了分析和比较。从第一次神经系统表现(神经系统发作组,包括 669 名患者)和诊断(诊断组,包括 590 名患者)使用针对各种协变量调整的 Cox 比例风险模型。总体而言,神经系统发作组的 384 名 (57.4%) 患者和诊断组的 329 名 (55.8%) 患者接受了米格司他治疗。Miglustat 治疗与两组的死亡率风险显着降低相关(整个神经系统发作组,风险比 [HR] = 0.51;整个诊断组,HR = 0.44;两者P <.001)。在所有神经系统发病年龄亚组(HRs = 0.3 至 0.7)中一致观察到这种效果,并且对于两组中晚期婴儿发病患者具有统计学意义(神经系统发病组,HR = 0.36,P < .05 ;诊断组,HR = 0.32,P < .01)和仅诊断组的青少年发病患者(HR = 0.30,P < .05)。尽管数据的局限性促使我们谨慎解释,但研究结果与米格司他对 NP-C 患者生存的有益影响一致。



























 京公网安备 11010802027423号
京公网安备 11010802027423号