当前位置:
X-MOL 学术
›
Nanoscale Horiz.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
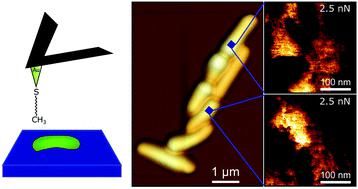
Fast chemical force microscopy demonstrates that glycopeptidolipids define nanodomains of varying hydrophobicity on mycobacteria.
Nanoscale Horizons ( IF 9.7 ) Pub Date : 2020-04-21 , DOI: 10.1039/c9nh00736a Albertus Viljoen 1 , Felipe Viela , Laurent Kremer , Yves F Dufrêne
Nanoscale Horizons ( IF 9.7 ) Pub Date : 2020-04-21 , DOI: 10.1039/c9nh00736a Albertus Viljoen 1 , Felipe Viela , Laurent Kremer , Yves F Dufrêne
Affiliation
|
Mycobacterium abscessus is an emerging multidrug-resistant bacterial pathogen causing severe lung infections in cystic fibrosis patients. A remarkable trait of this mycobacterial species is its ability to form morphologically smooth (S) and rough (R) colonies. The S-to-R transition is caused by the loss of glycopeptidolipids (GPLs) in the outer layer of the cell envelope and correlates with an increase in cording and virulence. Despite the physiological and medical importance of this morphological transition, whether it involves changes in cell surface properties remains unknown. Herein, we combine recently developed quantitative imaging (QI) atomic force microscopy (AFM) with hydrophobic tips to quantitatively map the surface structure and hydrophobicity of M. abscessus at high spatiotemporal resolution, and to assess how these properties are modulated by the S-to-R transition and by treatment with an inhibitor of the mycolic acid transporter MmpL3. We discover that loss of GPLs leads to major modifications in surface hydrophobicity, without any apparent change in cell surface ultrastructure. While R bacilli are homogeneously hydrophobic, S bacilli feature unusual variations of nanoscale hydrophobic properties. These previously undescribed cell surface nanodomains are likely to play critical roles in bacterial adhesion, aggregation, phenotypic heterogeneity and transmission, and in turn in virulence and pathogenicity. Our study also suggests that MmpL3 inhibitors show promise in nanomedicine as chemotherapeutic agents to interfere with the highly hydrophobic nature of the mycobacterial cell wall. The advantages of QI-AFM with hydrophobic tips are the ability to map chemical and structural properties simultaneously and at high resolution, applicable to a wide range of biosystems.
中文翻译:

快速化学力显微镜显示,糖肽脂质定义了分枝杆菌上疏水性不同的纳米域。
脓肿分枝杆菌是一种新兴的多药耐药细菌病原体,可在囊性纤维化患者中引起严重的肺部感染。该分枝杆菌物种的显着特征是其形成形态上光滑的(S)和粗糙(R)菌落的能力。S到R的过渡是由细胞被膜外层糖肽脂质(GPL)的丢失引起的,并且与绳索和毒力的增加有关。尽管这种形态学转变在生理和医学上都很重要,但是否涉及细胞表面特性的变化仍然未知。在这里,我们结合最近开发的定量成像(QI)原子力显微镜(AFM)与疏水性尖端,以定量地绘制脓肿的表面结构和疏水性在高时空分辨率下,并评估这些特性如何通过S-R过渡和霉菌酸转运蛋白MmpL3抑制剂的处理而得到调节。我们发现GPL的损失导致表面疏水性的重大修改,而细胞表面超微结构没有任何明显的变化。R杆菌具有均一的疏水性,而S细菌具有纳米级疏水性的异常变化。这些先前未描述的细胞表面纳米域可能在细菌粘附,聚集,表型异质性和传播,进而在毒力和致病性中起关键作用。我们的研究还表明,MmpL3抑制剂在纳米药物中有望作为化学治疗剂来干扰分枝杆菌细胞壁的高度疏水性。
更新日期:2020-04-21
中文翻译:

快速化学力显微镜显示,糖肽脂质定义了分枝杆菌上疏水性不同的纳米域。
脓肿分枝杆菌是一种新兴的多药耐药细菌病原体,可在囊性纤维化患者中引起严重的肺部感染。该分枝杆菌物种的显着特征是其形成形态上光滑的(S)和粗糙(R)菌落的能力。S到R的过渡是由细胞被膜外层糖肽脂质(GPL)的丢失引起的,并且与绳索和毒力的增加有关。尽管这种形态学转变在生理和医学上都很重要,但是否涉及细胞表面特性的变化仍然未知。在这里,我们结合最近开发的定量成像(QI)原子力显微镜(AFM)与疏水性尖端,以定量地绘制脓肿的表面结构和疏水性在高时空分辨率下,并评估这些特性如何通过S-R过渡和霉菌酸转运蛋白MmpL3抑制剂的处理而得到调节。我们发现GPL的损失导致表面疏水性的重大修改,而细胞表面超微结构没有任何明显的变化。R杆菌具有均一的疏水性,而S细菌具有纳米级疏水性的异常变化。这些先前未描述的细胞表面纳米域可能在细菌粘附,聚集,表型异质性和传播,进而在毒力和致病性中起关键作用。我们的研究还表明,MmpL3抑制剂在纳米药物中有望作为化学治疗剂来干扰分枝杆菌细胞壁的高度疏水性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号