Nature Metabolism ( IF 20.8 ) Pub Date : 2020-04-21 , DOI: 10.1038/s42255-020-0195-8 Andrés Méndez-Lucas 1 , Wei Lin 1 , Paul C Driscoll 1 , Nathalie Legrave 1 , Laura Novellasdemunt 1 , Chencheng Xie 2 , Mark Charles 3 , Zena Wilson 4 , Neil P Jones 3 , Stephen Rayport 5, 6 , Manuel Rodríguez-Justo 7 , Vivian Li 1 , James I MacRae 1 , Nissim Hay 8 , Xin Chen 9 , Mariia Yuneva 1
|
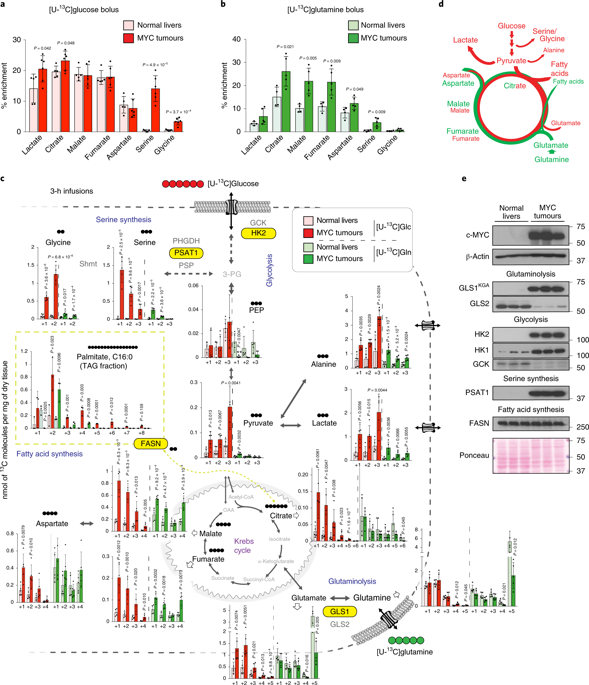
Plasticity of cancer metabolism can be a major obstacle to efficient targeting of tumour-specific metabolic vulnerabilities. Here, we identify the compensatory mechanisms following the inhibition of major pathways of central carbon metabolism in c-MYC-induced liver tumours. We find that, while inhibition of both glutaminase isoforms (Gls1 and Gls2) in tumours considerably delays tumourigenesis, glutamine catabolism continues, owing to the action of amidotransferases. Synergistic inhibition of both glutaminases and compensatory amidotransferases is required to block glutamine catabolism and proliferation of mouse and human tumour cells in vitro and in vivo. Gls1 deletion is also compensated for by glycolysis. Thus, co-inhibition of Gls1 and hexokinase 2 significantly affects Krebs cycle activity and tumour formation. Finally, the inhibition of biosynthesis of either serine (Psat1-KO) or fatty acid (Fasn-KO) is compensated for by uptake of circulating nutrients, and dietary restriction of both serine and glycine or fatty acids synergistically suppresses tumourigenesis. These results highlight the high flexibility of tumour metabolism and demonstrate that either pharmacological or dietary targeting of metabolic compensatory mechanisms can improve therapeutic outcomes.
中文翻译:

确定针对肿瘤代谢灵活性的策略。
癌症代谢的可塑性可能是有效靶向肿瘤特异性代谢脆弱性的主要障碍。在这里,我们确定了在 c-MYC 诱导的肝肿瘤中抑制中央碳代谢的主要途径后的补偿机制。我们发现,虽然抑制两种谷氨酰胺酶亚型(Gls 1 和Gls2) 在肿瘤中显着延迟肿瘤发生,由于氨基转移酶的作用,谷氨酰胺分解代谢继续。谷氨酰胺酶和代偿性氨基转移酶的协同抑制是在体外和体内阻断小鼠和人类肿瘤细胞的谷氨酰胺分解代谢和增殖所必需的。Gls1 缺失也通过糖酵解得到补偿。因此,Gls1 和己糖激酶 2 的共同抑制显着影响克雷布斯循环活性和肿瘤形成。最后,抑制丝氨酸 ( Psat1 -KO) 或脂肪酸 ( Fasn-KO) 通过摄取循环营养素来补偿,并且丝氨酸和甘氨酸或脂肪酸的饮食限制协同抑制肿瘤发生。这些结果突出了肿瘤代谢的高度灵活性,并证明代谢补偿机制的药理学或饮食靶向都可以改善治疗结果。


























 京公网安备 11010802027423号
京公网安备 11010802027423号