当前位置:
X-MOL 学术
›
J. Struct. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structure of human DPEP3 in complex with the SC-003 antibody Fab fragment reveals basis for lack of dipeptidase activity.
Journal of Structural Biology ( IF 3 ) Pub Date : 2020-04-20 , DOI: 10.1016/j.jsb.2020.107512 Kristyn Hayashi 1 , Kenton L Longenecker 2 , Patrick Koenig 1 , Aditi Prashar 1 , Johannes Hampl 1 , Vincent Stoll 2 , Sandro Vivona 3
Journal of Structural Biology ( IF 3 ) Pub Date : 2020-04-20 , DOI: 10.1016/j.jsb.2020.107512 Kristyn Hayashi 1 , Kenton L Longenecker 2 , Patrick Koenig 1 , Aditi Prashar 1 , Johannes Hampl 1 , Vincent Stoll 2 , Sandro Vivona 3
Affiliation
|
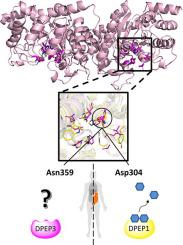
Dipeptidase 3 (DPEP3) is one of three glycosylphosphatidylinositol-anchored metallopeptidases potentially involved in the hydrolytic metabolism of dipeptides. While its exact biological function is not clear, DPEP3 expression is normally limited to testis , but can be elevated in ovarian cancer. Antibody drug conjugates targeting DPEP3 have shown efficacy in preclinical models with a pyrrolobenzodiazepine conjugate, SC-003, dosed in a phase I clinical trial (NCT02539719). Here we reveal the novel atomic structure of DPEP3 alone and in complex with the SC-003 Fab fragment at 1.8 and 2.8 Å, respectively. The structure of DPEP3/SC-003 Fab complex reveals an eighteen-residue epitope across the DPEP3 dimerization interface distinct from the enzymatic active site. DPEP1 and DPEP3 extracellular domains share a conserved, dimeric TIM (β/α)8-barrel fold, consistent with 49% sequence identity. However, DPEP3 diverges from DPEP1 and DPEP2 in key positions of its active site: a histidine to tyrosine variation at position 269 reduces affinity for the β zinc and may cause substrate steric hindrance, whereas an aspartate to asparagine change at position 359 abolishes activation of the nucleophilic water/hydroxide, resulting in no in vitro activity against a variety of dipeptides and biological substrates (imipenem, leukotriene D4 and cystinyl-bis-glycine). Hence DPEP3, unlike DPEP1 and DPEP2, may require an activating co-factor in vivo or may remain an inactive, degenerate enzyme. This report sheds light on the structural discriminants between active and inactive membrane dipeptidases and provides a benchmark to characterize current and future DPEP3-targeted therapeutic approaches.
中文翻译:

与 SC-003 抗体 Fab 片段复合的人 DPEP3 结构揭示了缺乏二肽酶活性的基础。
二肽酶 3 (DPEP3) 是三种糖基磷脂酰肌醇锚定的金属肽酶之一,可能参与二肽的水解代谢。虽然其确切的生物学功能尚不清楚,但 DPEP3 表达通常仅限于睾丸,但可以在卵巢癌中升高。靶向 DPEP3 的抗体药物偶联物已在 I 期临床试验 (NCT02539719) 中给药的吡咯并苯二氮卓偶联物 SC-003 的临床前模型中显示出疗效。在这里,我们分别揭示了 DPEP3 单独和与 SC-003 Fab 片段在 1.8 和 2.8 Å 处复合的新原子结构。DPEP3/SC-003 Fab 复合物的结构在 DPEP3 二聚化界面上显示了一个与酶活性位点不同的 18 个残基表位。DPEP1 和 DPEP3 细胞外结构域共享一个保守的二聚体 TIM (β/α)8 桶折叠,与 49% 的序列同一性一致。然而,DPEP3 在其活性位点的关键位置与 DPEP1 和 DPEP2 不同:269 位组氨酸到酪氨酸的变化降低了对 β 锌的亲和力并可能导致底物位阻,而 359 位的天冬氨酸到天冬酰胺的变化消除了亲核水/氢氧化物,导致对多种二肽和生物底物(亚胺培南、白三烯 D4 和胱氨酸双甘氨酸)没有体外活性。因此,与 DPEP1 和 DPEP2 不同,DPEP3 可能需要体内激活辅因子,或者可能保持无活性的简并酶。该报告阐明了活性和非活性膜二肽酶之间的结构区别,并提供了表征当前和未来 DPEP3 靶向治疗方法的基准。
更新日期:2020-04-22
中文翻译:

与 SC-003 抗体 Fab 片段复合的人 DPEP3 结构揭示了缺乏二肽酶活性的基础。
二肽酶 3 (DPEP3) 是三种糖基磷脂酰肌醇锚定的金属肽酶之一,可能参与二肽的水解代谢。虽然其确切的生物学功能尚不清楚,但 DPEP3 表达通常仅限于睾丸,但可以在卵巢癌中升高。靶向 DPEP3 的抗体药物偶联物已在 I 期临床试验 (NCT02539719) 中给药的吡咯并苯二氮卓偶联物 SC-003 的临床前模型中显示出疗效。在这里,我们分别揭示了 DPEP3 单独和与 SC-003 Fab 片段在 1.8 和 2.8 Å 处复合的新原子结构。DPEP3/SC-003 Fab 复合物的结构在 DPEP3 二聚化界面上显示了一个与酶活性位点不同的 18 个残基表位。DPEP1 和 DPEP3 细胞外结构域共享一个保守的二聚体 TIM (β/α)8 桶折叠,与 49% 的序列同一性一致。然而,DPEP3 在其活性位点的关键位置与 DPEP1 和 DPEP2 不同:269 位组氨酸到酪氨酸的变化降低了对 β 锌的亲和力并可能导致底物位阻,而 359 位的天冬氨酸到天冬酰胺的变化消除了亲核水/氢氧化物,导致对多种二肽和生物底物(亚胺培南、白三烯 D4 和胱氨酸双甘氨酸)没有体外活性。因此,与 DPEP1 和 DPEP2 不同,DPEP3 可能需要体内激活辅因子,或者可能保持无活性的简并酶。该报告阐明了活性和非活性膜二肽酶之间的结构区别,并提供了表征当前和未来 DPEP3 靶向治疗方法的基准。



























 京公网安备 11010802027423号
京公网安备 11010802027423号