Communications Chemistry ( IF 5.9 ) Pub Date : 2020-04-20 , DOI: 10.1038/s42004-020-0295-0 Lei Ke 1 , Zhilong Chen 1
|
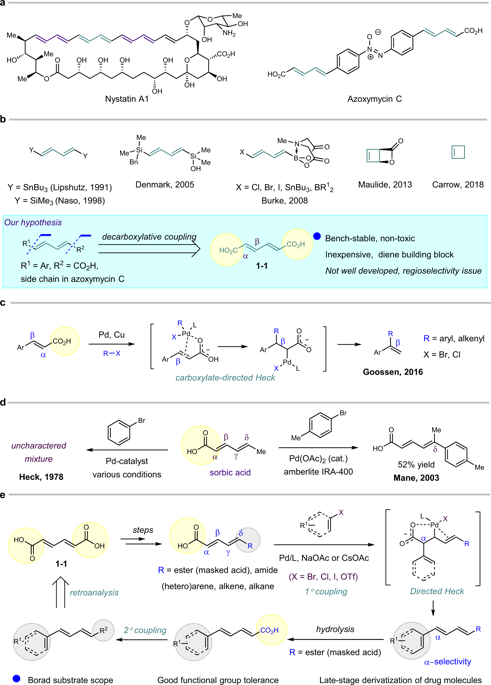
The concise construction of diene scaffolds is quite useful in the synthesis of polyenes. Many diene building blocks have been developed based on Suzuki, Still and Hiyama couplings. Herein, the commercially available and environmentally friendly compound dienedioic acid is used as a diene building block. Broad substrate scope, good functional group tolerance, and late-stage derivatization of complex drug molecules are achieved. Different moieties can be conveniently introduced to both sides. Piperine and the methyl ester of azoxymycin C are each prepared in three steps. Additionally, one product shows promising anticancer activities in leukemia K562 and MV-4-11 cells. Mechanistic studies indicate that the reaction proceeds through a Heck-decarboxylate coupling procedure, and the carboxylic group acts as a directing group to promote the reaction and control regioselectivity. Our research suggests that dienedioic acid can serve as a good alternative for diene preparation via a directed Heck-decarboxylate coupling.
中文翻译:

通过定向 Heck-脱羧偶联作为有用的二烯结构单元的二烯二酸
二烯支架的简明结构在多烯的合成中非常有用。许多二烯结构单元都是基于 Suzuki、Still 和 Hiyama 联轴器开发的。在此,使用市售且环保的化合物二烯二酸作为二烯结构单元。实现了广泛的底物范围、良好的官能团耐受性和复杂药物分子的后期衍生化。可以方便地将不同的部分引入到两侧。胡椒碱和azoxymycin C甲酯分别分三步制备。此外,一种产品在白血病 K562 和 MV-4-11 细胞中显示出有前途的抗癌活性。机理研究表明反应通过 Heck-脱羧偶联程序进行,羧基作为导向基团促进反应和控制区域选择性。我们的研究表明,二烯二酸可以作为通过定向 Heck-脱羧偶联制备二烯的良好替代品。



























 京公网安备 11010802027423号
京公网安备 11010802027423号