当前位置:
X-MOL 学术
›
Biotechnol. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crystal Contact Engineering Enables Efficient Capture and Purification of an Oxidoreductase by Technical Crystallization.
Biotechnology Journal ( IF 4.7 ) Pub Date : 2020-04-17 , DOI: 10.1002/biot.202000010 Phillip Grob 1 , Max Huber 1 , Brigitte Walla 1 , Johannes Hermann 1 , Robert Janowski 2 , Dierk Niessing 2, 3 , Dariusch Hekmat 1 , Dirk Weuster-Botz 1
Biotechnology Journal ( IF 4.7 ) Pub Date : 2020-04-17 , DOI: 10.1002/biot.202000010 Phillip Grob 1 , Max Huber 1 , Brigitte Walla 1 , Johannes Hermann 1 , Robert Janowski 2 , Dierk Niessing 2, 3 , Dariusch Hekmat 1 , Dirk Weuster-Botz 1
Affiliation
|
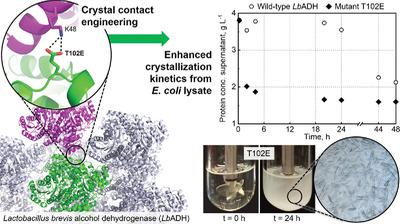
Technical crystallization is an attractive method to purify recombinant proteins. However, it is rarely applied due to the limited crystallizability of many proteins. To overcome this limitation, single amino acid exchanges are rationally introduced to enhance intermolecular interactions at the crystal contacts of the industrially relevant biocatalyst Lactobacillus brevis alcohol dehydrogenase (LbADH). The wildtype (WT) and the best crystallizing and enzymatically active LbADH mutants K32A, D54F, Q126H, and T102E are produced with Escherichia coli and subsequently crystallized from cell lysate in stirred mL‐crystallizers. Notwithstanding the high host cell protein (HCP) concentrations in the lysate, all mutants crystallize significantly faster than the WT. Combinations of mutations result in double mutants with faster crystallization kinetics than the respective single mutants, demonstrating a synergetic effect. The almost entire depletion of the soluble LbADH fraction at crystallization equilibrium is observed, proving high yields. The HCP concentration is reduced to below 0.5% after crystal dissolution and recrystallization, and thus a 100‐fold HCP reduction is achieved after two successive crystallization steps. The combination of fast kinetics, high yields, and high target protein purity highlights the potential of crystal contact engineering to transform technical crystallization into an efficient protein capture and purification step in biotechnological downstream processes.
中文翻译:

晶体接触工程技术可以通过技术结晶有效地捕获和纯化氧化还原酶。
技术结晶是纯化重组蛋白的一种有吸引力的方法。但是,由于许多蛋白质的结晶性有限,因此很少应用。为了克服此限制,合理地引入了单个氨基酸交换,以增强工业相关生物催化剂短乳杆菌乙醇脱氢酶(Lb ADH)的晶体接触处的分子间相互作用。野生型(WT)和具有最佳结晶和酶活性的Lb ADH突变体K32A,D54F,Q126H和T102E由大肠杆菌生产然后在搅拌的mL结晶器中从细胞裂解物中结晶出来。尽管裂解液中的宿主细胞蛋白(HCP)浓度较高,但所有突变体的结晶速度均显着高于WT。突变的组合导致双突变体具有比相应的单个突变体更快的结晶动力学,这表明了协同作用。可溶性Lb几乎全部耗尽观察到处于结晶平衡的ADH馏分,证明了高产率。晶体溶解和重结晶后,HCP浓度降低到0.5%以下,因此在两个连续的结晶步骤后,HCP降低了100倍。快速动力学,高产率和高目标蛋白质纯度的结合突出了晶体接触工程技术的潜力,可以将技术结晶转化为生物技术下游工艺中有效的蛋白质捕获和纯化步骤。
更新日期:2020-04-17
中文翻译:

晶体接触工程技术可以通过技术结晶有效地捕获和纯化氧化还原酶。
技术结晶是纯化重组蛋白的一种有吸引力的方法。但是,由于许多蛋白质的结晶性有限,因此很少应用。为了克服此限制,合理地引入了单个氨基酸交换,以增强工业相关生物催化剂短乳杆菌乙醇脱氢酶(Lb ADH)的晶体接触处的分子间相互作用。野生型(WT)和具有最佳结晶和酶活性的Lb ADH突变体K32A,D54F,Q126H和T102E由大肠杆菌生产然后在搅拌的mL结晶器中从细胞裂解物中结晶出来。尽管裂解液中的宿主细胞蛋白(HCP)浓度较高,但所有突变体的结晶速度均显着高于WT。突变的组合导致双突变体具有比相应的单个突变体更快的结晶动力学,这表明了协同作用。可溶性Lb几乎全部耗尽观察到处于结晶平衡的ADH馏分,证明了高产率。晶体溶解和重结晶后,HCP浓度降低到0.5%以下,因此在两个连续的结晶步骤后,HCP降低了100倍。快速动力学,高产率和高目标蛋白质纯度的结合突出了晶体接触工程技术的潜力,可以将技术结晶转化为生物技术下游工艺中有效的蛋白质捕获和纯化步骤。



























 京公网安备 11010802027423号
京公网安备 11010802027423号