当前位置:
X-MOL 学术
›
Cell Death Discov.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A comparative pharmaco-metabolomic study of glutaminase inhibitors in glioma stem-like cells confirms biological effectiveness but reveals differences in target-specificity.
Cell Death Discovery ( IF 7 ) Pub Date : 2020-04-16 , DOI: 10.1038/s41420-020-0258-3 Katharina Koch 1 , Rudolf Hartmann 2 , Julia Tsiampali 1 , Constanze Uhlmann 1 , Ann-Christin Nickel 1 , Xiaoling He 3 , Marcel A Kamp 1 , Michael Sabel 1 , Roger A Barker 3 , Hans-Jakob Steiger 1 , Daniel Hänggi 1 , Dieter Willbold 2, 4 , Jaroslaw Maciaczyk 1, 5 , Ulf D Kahlert 1, 6
Cell Death Discovery ( IF 7 ) Pub Date : 2020-04-16 , DOI: 10.1038/s41420-020-0258-3 Katharina Koch 1 , Rudolf Hartmann 2 , Julia Tsiampali 1 , Constanze Uhlmann 1 , Ann-Christin Nickel 1 , Xiaoling He 3 , Marcel A Kamp 1 , Michael Sabel 1 , Roger A Barker 3 , Hans-Jakob Steiger 1 , Daniel Hänggi 1 , Dieter Willbold 2, 4 , Jaroslaw Maciaczyk 1, 5 , Ulf D Kahlert 1, 6
Affiliation
|
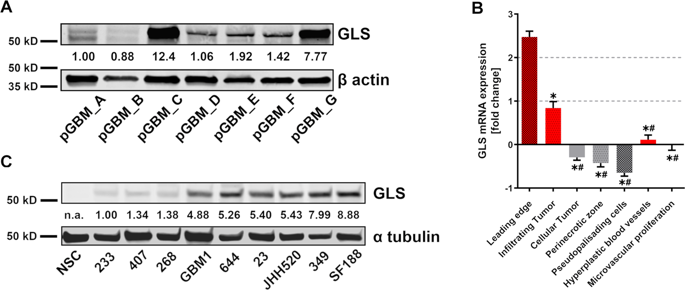
Cancer cells upregulate anabolic processes to maintain high rates of cellular turnover. Limiting the supply of macromolecular precursors by targeting enzymes involved in biosynthesis is a promising strategy in cancer therapy. Several tumors excessively metabolize glutamine to generate precursors for nonessential amino acids, nucleotides, and lipids, in a process called glutaminolysis. Here we show that pharmacological inhibition of glutaminase (GLS) eradicates glioblastoma stem-like cells (GSCs), a small cell subpopulation in glioblastoma (GBM) responsible for therapy resistance and tumor recurrence. Treatment with small molecule inhibitors compound 968 and CB839 effectively diminished cell growth and in vitro clonogenicity of GSC neurosphere cultures. However, our pharmaco-metabolic studies revealed that only CB839 inhibited GLS enzymatic activity thereby limiting the influx of glutamine derivates into the TCA cycle. Nevertheless, the effects of both inhibitors were highly GLS specific, since treatment sensitivity markedly correlated with GLS protein expression. Strikingly, we found GLS overexpressed in in vitro GSC models as compared with neural stem cells (NSC). Moreover, our study demonstrates the usefulness of in vitro pharmaco-metabolomics to score target specificity of compounds thereby refining drug development and risk assessment.
中文翻译:

神经胶质瘤干细胞样谷氨酰胺酶抑制剂的比较药物代谢组学研究证实了其生物学有效性,但揭示了靶点特异性的差异。
癌细胞上调合成代谢过程以维持高细胞更新率。通过靶向参与生物合成的酶来限制大分子前体的供应是癌症治疗中一种有前景的策略。一些肿瘤过度代谢谷氨酰胺,产生非必需氨基酸、核苷酸和脂质的前体,这一过程称为谷氨酰胺分解。在这里,我们表明,谷氨酰胺酶(GLS)的药理学抑制可以根除胶质母细胞瘤干样细胞(GSC),这是胶质母细胞瘤(GBM)中负责治疗耐药和肿瘤复发的小细胞亚群。用小分子抑制剂化合物 968 和 CB839 处理可有效减少 GSC 神经球培养物的细胞生长和体外克隆形成。然而,我们的药物代谢研究表明,只有 CB839 抑制 GLS 酶活性,从而限制谷氨酰胺衍生物流入 TCA 循环。然而,两种抑制剂的作用均具有高度 GLS 特异性,因为治疗敏感性与 GLS 蛋白表达显着相关。引人注目的是,我们发现与神经干细胞 (NSC) 相比,GLS 在体外 GSC 模型中过度表达。此外,我们的研究证明了体外药物代谢组学对化合物的目标特异性进行评分的有用性,从而改进了药物开发和风险评估。
更新日期:2020-04-24
中文翻译:

神经胶质瘤干细胞样谷氨酰胺酶抑制剂的比较药物代谢组学研究证实了其生物学有效性,但揭示了靶点特异性的差异。
癌细胞上调合成代谢过程以维持高细胞更新率。通过靶向参与生物合成的酶来限制大分子前体的供应是癌症治疗中一种有前景的策略。一些肿瘤过度代谢谷氨酰胺,产生非必需氨基酸、核苷酸和脂质的前体,这一过程称为谷氨酰胺分解。在这里,我们表明,谷氨酰胺酶(GLS)的药理学抑制可以根除胶质母细胞瘤干样细胞(GSC),这是胶质母细胞瘤(GBM)中负责治疗耐药和肿瘤复发的小细胞亚群。用小分子抑制剂化合物 968 和 CB839 处理可有效减少 GSC 神经球培养物的细胞生长和体外克隆形成。然而,我们的药物代谢研究表明,只有 CB839 抑制 GLS 酶活性,从而限制谷氨酰胺衍生物流入 TCA 循环。然而,两种抑制剂的作用均具有高度 GLS 特异性,因为治疗敏感性与 GLS 蛋白表达显着相关。引人注目的是,我们发现与神经干细胞 (NSC) 相比,GLS 在体外 GSC 模型中过度表达。此外,我们的研究证明了体外药物代谢组学对化合物的目标特异性进行评分的有用性,从而改进了药物开发和风险评估。



























 京公网安备 11010802027423号
京公网安备 11010802027423号