当前位置:
X-MOL 学术
›
BBA Proteins Proteom.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Separation of D-amino acid-containing peptide phenylseptin using 3,3'-phenyl-1,1'-binaphthyl-18-crown-6-ether columns.
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 3.2 ) Pub Date : 2020-04-13 , DOI: 10.1016/j.bbapap.2020.140429 Izuru Kawamura 1 , Batsaikhan Mijiddorj 2 , Yohei Kayano 3 , Yuta Matsuo 4 , Yumi Ozawa 4 , Kazuyoshi Ueda 4 , Hisako Sato 5
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 3.2 ) Pub Date : 2020-04-13 , DOI: 10.1016/j.bbapap.2020.140429 Izuru Kawamura 1 , Batsaikhan Mijiddorj 2 , Yohei Kayano 3 , Yuta Matsuo 4 , Yumi Ozawa 4 , Kazuyoshi Ueda 4 , Hisako Sato 5
Affiliation
|
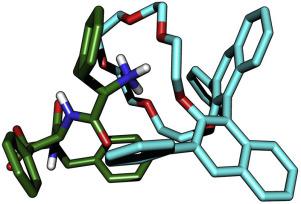
Several D-amino acid-containing peptides (DAACPs) with antimicrobial, cardio-excitatory, or neuronal activities have been found in several species. Here, we demonstrated the chiral separation of the antimicrobial peptide diastereomers, D-phenylseptin and L-phenylseptin using (S) and (R) 3,3'-phenyl-1,1'-binaphthyl-18-crown-6-ether columns (CR-I (+) and CR-I (-), respectively) and also investigated the underlying mechanism. First, using D-amino acid-containing tripeptide Phe-Phe-Phe-OH, we found that CR-I (+) could be used to recognize diastereomeric tripeptides containing an L-amino acid as the first residue. On the contrary, CR-I (-) enabled separation of a series of diastereomers with D-amino acid as the first residue. Therefore, we achieved separation of the stereoisomers using the chiral columns depending on the position of the D- amino acid in the peptide and demonstrated the orthogonality of separations of the chiral columns. Then, using CR-I (+), we separated amphibian antimicrobial peptide diastereomers, L- and D-phenylseptin, which have the sequences, L-Phe-L-Phe-L-Phe and L-Phe-D-Phe-L-Phe at their N-termini, respectively. In order to understand the host-guest interactions, we performed molecular dynamics simulations for L-Phe-L-Phe-L-Phe tripeptide-CR-I molecule complex systems. Three hydrogen bonds between the N-terminal amine group -NH3+ and the crown ether oxygens were the dominant interactions. The hydrophobic interactions between phenyl-rings in the chiral selector unit of CR-I (+) and the side chains of 2nd and 3rd residues of the peptide also contributed to the affinity. Our results show that the CR-I (+)-column can be applied for the separation of endogenous DAACPs generated by the post-translational modification.
中文翻译:

使用3,3'-苯基-1,1'-联萘-18-冠-6-醚色谱柱分离D-氨基酸的肽苯基肽。
在几种物种中发现了几种具有抗菌,心脏兴奋或神经元活性的含D-氨基酸的肽(DAACP)。在这里,我们展示了使用(S)和(R)3,3'-苯基-1,1'-联萘基-18-冠-6-醚色谱柱对抗菌肽非对映异构体D-苯基链霉菌素和L-苯基链菌素进行手性分离(分别为CR-1(+)和CR-1(-)),并且还研究了潜在的机制。首先,使用含D-氨基酸的三肽Phe-Phe-Phe-OH,我们发现CR-1(+)可用于识别含有L-氨基酸作为第一个残基的非对映三肽。相反,CR-1(-)能够分离一系列以D-氨基酸为第一个残基的非对映异构体。因此,我们根据肽中D-氨基酸的位置使用手性柱实现了立体异构体的分离,并证明了手性柱分离的正交性。然后,使用CR-I(+),分离具有序列L-Phe-L-Phe-L-Phe和L-Phe-D-Phe-L的两栖类抗菌肽非对映异构体L-和D-苯基丝氨酸。 -Phe分别位于其N末端。为了理解宿主-客体的相互作用,我们对L-Phe-L-Phe-L-Phe三肽-CR-1分子复合物系统进行了分子动力学模拟。N-末端胺基团-NH3 +与冠醚氧之间的三个氢键是主要的相互作用。CR-1(+)的手性选择器单元中的苯环与肽的第二和第三残基的侧链之间的疏水相互作用也有助于亲和力。我们的结果表明,CR-1(+)柱可用于分离由翻译后修饰产生的内源性DAACP。
更新日期:2020-04-20
中文翻译:

使用3,3'-苯基-1,1'-联萘-18-冠-6-醚色谱柱分离D-氨基酸的肽苯基肽。
在几种物种中发现了几种具有抗菌,心脏兴奋或神经元活性的含D-氨基酸的肽(DAACP)。在这里,我们展示了使用(S)和(R)3,3'-苯基-1,1'-联萘基-18-冠-6-醚色谱柱对抗菌肽非对映异构体D-苯基链霉菌素和L-苯基链菌素进行手性分离(分别为CR-1(+)和CR-1(-)),并且还研究了潜在的机制。首先,使用含D-氨基酸的三肽Phe-Phe-Phe-OH,我们发现CR-1(+)可用于识别含有L-氨基酸作为第一个残基的非对映三肽。相反,CR-1(-)能够分离一系列以D-氨基酸为第一个残基的非对映异构体。因此,我们根据肽中D-氨基酸的位置使用手性柱实现了立体异构体的分离,并证明了手性柱分离的正交性。然后,使用CR-I(+),分离具有序列L-Phe-L-Phe-L-Phe和L-Phe-D-Phe-L的两栖类抗菌肽非对映异构体L-和D-苯基丝氨酸。 -Phe分别位于其N末端。为了理解宿主-客体的相互作用,我们对L-Phe-L-Phe-L-Phe三肽-CR-1分子复合物系统进行了分子动力学模拟。N-末端胺基团-NH3 +与冠醚氧之间的三个氢键是主要的相互作用。CR-1(+)的手性选择器单元中的苯环与肽的第二和第三残基的侧链之间的疏水相互作用也有助于亲和力。我们的结果表明,CR-1(+)柱可用于分离由翻译后修饰产生的内源性DAACP。


























 京公网安备 11010802027423号
京公网安备 11010802027423号