Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanism of CcdA-Mediated Rejuvenation of DNA Gyrase.
Structure ( IF 5.7 ) Pub Date : 2020-04-14 , DOI: 10.1016/j.str.2020.03.006 Nilesh K Aghera 1 , Jyothi Prabha 1 , Himani Tandon 1 , Gopinath Chattopadhyay 1 , Sneha Vishwanath 1 , Narayanaswamy Srinivasan 1 , Raghavan Varadarajan 2
Structure ( IF 5.7 ) Pub Date : 2020-04-14 , DOI: 10.1016/j.str.2020.03.006 Nilesh K Aghera 1 , Jyothi Prabha 1 , Himani Tandon 1 , Gopinath Chattopadhyay 1 , Sneha Vishwanath 1 , Narayanaswamy Srinivasan 1 , Raghavan Varadarajan 2
Affiliation
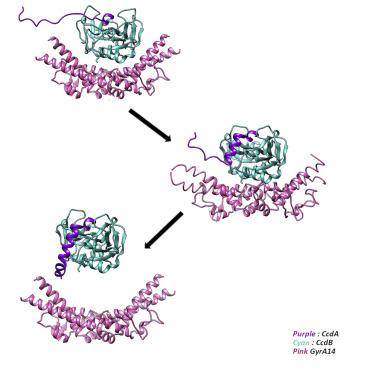
|
Most biological processes involve formation of transient complexes where binding of a ligand allosterically modulates function. The ccd toxin-antitoxin system is involved in plasmid maintenance and bacterial persistence. The CcdA antitoxin accelerates dissociation of CcdB from its complex with DNA gyrase, binds and neutralizes CcdB, but the mechanistic details are unclear. Using a series of experimental and computational approaches, we demonstrate the formation of transient ternary and quaternary CcdA:CcdB:gyrase complexes and delineate the molecular steps involved in the rejuvenation process. Binding of region 61-72 of CcdA to CcdB induces the vital structural and dynamic changes required to facilitate dissociation from gyrase, region 50-60 enhances the dissociation process through additional allosteric effects, and segment 37-49 prevents gyrase rebinding. This study provides insights into molecular mechanisms responsible for recovery of CcdB-poisoned cells from a persister-like state. Similar methodology can be used to characterize other important transient, macromolecular complexes.
中文翻译:

CcdA介导的DNA促旋酶复兴的机制。
大多数生物学过程都涉及形成瞬态复合物,其中配体的结合会变构地调节功能。ccd毒素-抗毒素系统参与质粒的维持和细菌的持久性。CcdA抗毒素可加速CcdB从其与DNA促旋酶的复合物中解离,结合并中和CcdB,但机制细节尚不清楚。使用一系列实验和计算方法,我们证明了瞬态三元和四元CcdA:CcdB:gyrase复合物的形成,并描绘了复兴过程中涉及的分子步骤。CcdA的61-72区与CcdB的结合诱导了促进从促旋酶解离所需的重要结构和动态变化,区域50-60通过其他变构作用增强了解离过程,37-49节阻止了促旋酶的重新结合。这项研究提供了从持久性状态恢复CcdB中毒细胞的分子机制的见解。相似的方法可用于表征其他重要的瞬态大分子复合物。
更新日期:2020-04-14
中文翻译:

CcdA介导的DNA促旋酶复兴的机制。
大多数生物学过程都涉及形成瞬态复合物,其中配体的结合会变构地调节功能。ccd毒素-抗毒素系统参与质粒的维持和细菌的持久性。CcdA抗毒素可加速CcdB从其与DNA促旋酶的复合物中解离,结合并中和CcdB,但机制细节尚不清楚。使用一系列实验和计算方法,我们证明了瞬态三元和四元CcdA:CcdB:gyrase复合物的形成,并描绘了复兴过程中涉及的分子步骤。CcdA的61-72区与CcdB的结合诱导了促进从促旋酶解离所需的重要结构和动态变化,区域50-60通过其他变构作用增强了解离过程,37-49节阻止了促旋酶的重新结合。这项研究提供了从持久性状态恢复CcdB中毒细胞的分子机制的见解。相似的方法可用于表征其他重要的瞬态大分子复合物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号