当前位置:
X-MOL 学术
›
Nat. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dynamics of oligomer populations formed during the aggregation of Alzheimer's Aβ42 peptide.
Nature Chemistry ( IF 21.8 ) Pub Date : 2020-04-13 , DOI: 10.1038/s41557-020-0452-1 Thomas C T Michaels 1, 2 , Andela Šarić 3, 4 , Samo Curk 3, 4, 5 , Katja Bernfur 6 , Paolo Arosio 7 , Georg Meisl 1 , Alexander J Dear 1, 2 , Samuel I A Cohen 1 , Christopher M Dobson 1 , Michele Vendruscolo 1 , Sara Linse 6 , Tuomas P J Knowles 1, 8
Nature Chemistry ( IF 21.8 ) Pub Date : 2020-04-13 , DOI: 10.1038/s41557-020-0452-1 Thomas C T Michaels 1, 2 , Andela Šarić 3, 4 , Samo Curk 3, 4, 5 , Katja Bernfur 6 , Paolo Arosio 7 , Georg Meisl 1 , Alexander J Dear 1, 2 , Samuel I A Cohen 1 , Christopher M Dobson 1 , Michele Vendruscolo 1 , Sara Linse 6 , Tuomas P J Knowles 1, 8
Affiliation
|
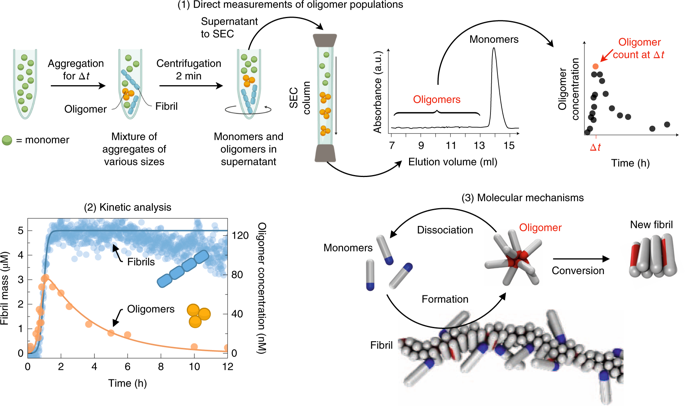
Oligomeric species populated during the aggregation of the Aβ42 peptide have been identified as potent cytotoxins linked to Alzheimer's disease, but the fundamental molecular pathways that control their dynamics have yet to be elucidated. By developing a general approach that combines theory, experiment and simulation, we reveal, in molecular detail, the mechanisms of Aβ42 oligomer dynamics during amyloid fibril formation. Even though all mature amyloid fibrils must originate as oligomers, we found that most Aβ42 oligomers dissociate into their monomeric precursors without forming new fibrils. Only a minority of oligomers converts into fibrillar structures. Moreover, the heterogeneous ensemble of oligomeric species interconverts on timescales comparable to those of aggregation. Our results identify fundamentally new steps that could be targeted by therapeutic interventions designed to combat protein misfolding diseases.
中文翻译:

在阿尔茨海默氏症Aβ42肽聚集过程中形成的低聚物种群动态。
在Aβ42肽聚集过程中居住的寡聚物种已被确定为与阿尔茨海默氏病相关的有效细胞毒素,但控制其动力学的基本分子途径尚未阐明。通过开发一种结合理论,实验和模拟的通用方法,我们在分子细节上揭示了淀粉样蛋白原纤维形成过程中Aβ42低聚物动力学的机制。尽管所有成熟的淀粉样蛋白原纤维都必须以低聚物形式产生,但我们发现大多数Aβ42低聚物均会解离成其单体前体,而不会形成新的原纤维。只有少数的低聚物转化为纤维状结构。此外,寡聚物种的异质集合在时间尺度上可以与聚合的时间尺度相互转换。
更新日期:2020-04-24
中文翻译:

在阿尔茨海默氏症Aβ42肽聚集过程中形成的低聚物种群动态。
在Aβ42肽聚集过程中居住的寡聚物种已被确定为与阿尔茨海默氏病相关的有效细胞毒素,但控制其动力学的基本分子途径尚未阐明。通过开发一种结合理论,实验和模拟的通用方法,我们在分子细节上揭示了淀粉样蛋白原纤维形成过程中Aβ42低聚物动力学的机制。尽管所有成熟的淀粉样蛋白原纤维都必须以低聚物形式产生,但我们发现大多数Aβ42低聚物均会解离成其单体前体,而不会形成新的原纤维。只有少数的低聚物转化为纤维状结构。此外,寡聚物种的异质集合在时间尺度上可以与聚合的时间尺度相互转换。


























 京公网安备 11010802027423号
京公网安备 11010802027423号