当前位置:
X-MOL 学术
›
Clin. Exp. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Changes in lung immune cells related to clinical outcome during treatment with infliximab for sarcoidosis.
Clinical & Experimental Immunology ( IF 4.6 ) Pub Date : 2020-04-10 , DOI: 10.1111/cei.13438 S Kullberg 1, 2 , N V Rivera 2 , M Abo Al Hayja 2 , J Grunewald 1, 2 , A Eklund 1, 2
Clinical & Experimental Immunology ( IF 4.6 ) Pub Date : 2020-04-10 , DOI: 10.1111/cei.13438 S Kullberg 1, 2 , N V Rivera 2 , M Abo Al Hayja 2 , J Grunewald 1, 2 , A Eklund 1, 2
Affiliation
|
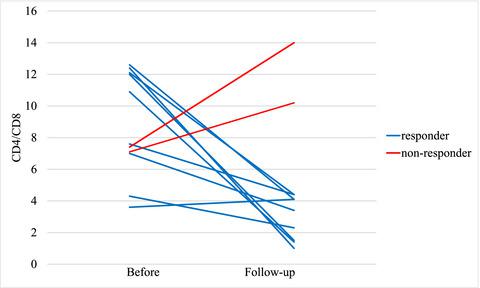
Pulmonary sarcoidosis is characterized by an exaggerated CD4+ T cell response and formation of non‐necrotizing granulomas. Tumour necrosis factor α (TNF‐α) is regarded as crucial for granuloma formation and TNF‐α inhibitors offer a third‐line treatment option for patients not responding to conventional treatment. However, not all patients benefit from treatment, and an optimal dose and treatment duration have not been established. Insight into the influence of TNF‐α inhibitors on lung immune cells may provide clues as to what drives inflammation in sarcoidosis and improve our understanding of treatment outcomes. To evaluate the effects of treatment with the TNF‐α inhibitor infliximab on lung immune cells and clinical features of the patients, 13 patients with sarcoidosis refractory to conventional treatment were assessed with bronchoalveolar lavage (BAL), spirometry and computerized tomography (CT) scan closely adjacent to the start of infliximab treatment. These investigations were repeated after 6 months of treatment. Treatment with TNF‐α inhibitor infliximab was well tolerated with no adverse events, except for one patient who developed a probable adverse event with liver toxicity. Ten patients were classified as responders, having a reduced CD4/CD8 ratio, a decreased percentage of CD4+ T cells expressing the activation marker CD69 and number of mast cells (P < 0·05 for all). The percentage of T regulatory cells (Tregs), defined as forkhead box P3+ CD4+ T cells decreased in most patients. In conclusion, six months of infliximab treatment in patients with sarcoidosis led to signs of decreased CD4+ T cell alveolitis and decreased mastocytosis in the lungs of responders.
中文翻译:

英夫利昔单抗结节病治疗期间与临床结果相关的肺免疫细胞变化。
肺结节病的特征是CD4 +T细胞反应和非坏死性肉芽肿的形成。肿瘤坏死因子α(TNF-α)被认为对肉芽肿形成至关重要,而TNF-α抑制剂为对常规治疗无反应的患者提供了三线治疗选择。但是,并非所有患者都能从治疗中受益,并且尚未确定最佳剂量和治疗持续时间。深入了解TNF-α抑制剂对肺免疫细胞的影响,可能会为结节病炎症驱动因素和我们对治疗结果的了解提供线索。为了评估TNF-α抑制剂英夫利昔单抗对肺部免疫细胞的治疗效果和患者的临床特征,对13例常规治疗难以治愈的结节病患者进行了支气管肺泡灌洗(BAL),肺活量测定和计算机断层扫描(CT)扫描与英夫利昔单抗治疗的开始紧密相邻。治疗6个月后重复进行这些检查。TNF-α抑制剂英夫利昔单抗的治疗耐受性良好,无不良事件,只有一名患者出现可能的肝毒性不良事件。十名患者被分类为缓解者,其CD4 / CD8比率降低,CD4百分比降低表达活化标记CD69和肥大细胞数量的+ T细胞(所有P <0·05)。在大多数患者中,T调节细胞(T regs)(定义为叉头盒P3 + CD4 + T细胞)的百分比下降。总之,对结节病患者进行英夫利昔单抗治疗六个月可导致应答者肺部CD4 + T细胞性肺泡炎减少和肥大细胞减少的迹象。
更新日期:2020-04-10
中文翻译:

英夫利昔单抗结节病治疗期间与临床结果相关的肺免疫细胞变化。
肺结节病的特征是CD4 +T细胞反应和非坏死性肉芽肿的形成。肿瘤坏死因子α(TNF-α)被认为对肉芽肿形成至关重要,而TNF-α抑制剂为对常规治疗无反应的患者提供了三线治疗选择。但是,并非所有患者都能从治疗中受益,并且尚未确定最佳剂量和治疗持续时间。深入了解TNF-α抑制剂对肺免疫细胞的影响,可能会为结节病炎症驱动因素和我们对治疗结果的了解提供线索。为了评估TNF-α抑制剂英夫利昔单抗对肺部免疫细胞的治疗效果和患者的临床特征,对13例常规治疗难以治愈的结节病患者进行了支气管肺泡灌洗(BAL),肺活量测定和计算机断层扫描(CT)扫描与英夫利昔单抗治疗的开始紧密相邻。治疗6个月后重复进行这些检查。TNF-α抑制剂英夫利昔单抗的治疗耐受性良好,无不良事件,只有一名患者出现可能的肝毒性不良事件。十名患者被分类为缓解者,其CD4 / CD8比率降低,CD4百分比降低表达活化标记CD69和肥大细胞数量的+ T细胞(所有P <0·05)。在大多数患者中,T调节细胞(T regs)(定义为叉头盒P3 + CD4 + T细胞)的百分比下降。总之,对结节病患者进行英夫利昔单抗治疗六个月可导致应答者肺部CD4 + T细胞性肺泡炎减少和肥大细胞减少的迹象。



























 京公网安备 11010802027423号
京公网安备 11010802027423号