Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The metabolic response to inflammation in astrocytes is regulated by nuclear factor-kappa B signaling.
Glia ( IF 6.2 ) Pub Date : 2020-04-10 , DOI: 10.1002/glia.23835 Josephine L Robb 1 , Nadia A Hammad 1 , Paul G Weightman Potter 1 , John K Chilton 1 , Craig Beall 1 , Kate L J Ellacott 1
Glia ( IF 6.2 ) Pub Date : 2020-04-10 , DOI: 10.1002/glia.23835 Josephine L Robb 1 , Nadia A Hammad 1 , Paul G Weightman Potter 1 , John K Chilton 1 , Craig Beall 1 , Kate L J Ellacott 1
Affiliation
|
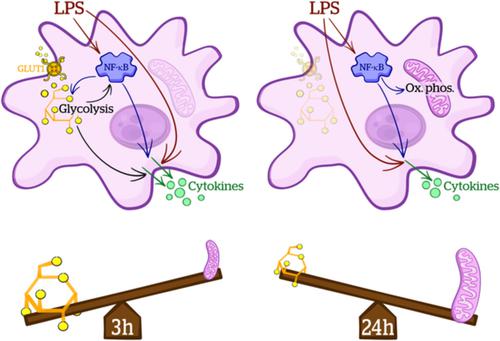
Inflammation and metabolism are intrinsically linked with inflammatory stimuli inducing metabolic changes in cells and, in turn, metabolic capacity determining cellular inflammatory responses. Although well characterized in peripheral immune cells there is comparatively less known about these “immunometabolic” responses in astrocytes. In this study, we tested the hypothesis that the astrocytic inflammatory response driven by nuclear factor‐kappa B (NF‐κB) signaling is dependent on glycolytic metabolism. Using mouse primary cortical astrocyte cultures, we assessed changes in cellular metabolism after exposure to lipopolysaccharide (LPS), with cytokine ELISAs and immunoblotting being used to measure inflammatory responses. Results indicate temporally distinct metabolic adaptations to pro‐inflammatory stimulation in astrocytes: 3 hr LPS treatment increased glycolysis but did not alter mitochondrial metabolism, while following 24 hr of LPS treatment we observed increased oxidative phosphorylation, and decreased glycolytic capacity and glucose uptake, partly due to reduced glucose transporter 1 expression. Inhibition of NF‐κB signaling with the IKK‐beta inhibitor TPCA‐1 prevented the LPS induced changes to glycolysis and oxidative phosphorylation. Furthermore, TPCA‐1 treatment altered both glycolysis and oxidative phosphorylation independently from inflammatory stimulation, indicating a role for NF‐κB signaling in regulation of basal metabolism in astrocytes. Inhibition of glycolysis with 2‐deoxyglucose significantly attenuated LPS‐induced cytokine release and NF‐κB phosphorylation, indicating that intact glycolysis is required for the full inflammatory response to LPS. Together our data indicate that astrocytes display immunometabolic responses to acute LPS stimulation which may represent a potential therapeutic target for neuroinflammatory disorders.
中文翻译:

星形胶质细胞对炎症的代谢反应受核因子-κB 信号的调节。
炎症和代谢与诱导细胞代谢变化的炎症刺激有着内在的联系,进而决定细胞炎症反应的代谢能力。尽管在外周免疫细胞中得到了很好的表征,但对星形胶质细胞中的这些“免疫代谢”反应知之甚少。在这项研究中,我们检验了由核因子-κB (NF-κB) 信号驱动的星形胶质细胞炎症反应依赖于糖酵解代谢的假设。使用小鼠原代皮质星形胶质细胞培养物,我们评估了暴露于脂多糖 (LPS) 后细胞代谢的变化,并使用细胞因子 ELISA 和免疫印迹来测量炎症反应。结果表明星形胶质细胞对促炎刺激的代谢适应在时间上不同:3 小时 LPS 处理增加了糖酵解,但没有改变线粒体代谢,而在 LPS 处理 24 小时后,我们观察到氧化磷酸化增加,糖酵解能力和葡萄糖摄取降低,部分原因是葡萄糖转运蛋白 1 表达减少。用 IKK-β 抑制剂 TPCA-1 抑制 NF-κB 信号可以防止 LPS 诱导的糖酵解和氧化磷酸化变化。此外,TPCA-1 治疗独立于炎症刺激改变糖酵解和氧化磷酸化,表明 NF-κB 信号在调节星形胶质细胞基础代谢中的作用。用 2-脱氧葡萄糖抑制糖酵解显着减弱了 LPS 诱导的细胞因子释放和 NF-κB 磷酸化,表明对 LPS 的完全炎症反应需要完整的糖酵解。
更新日期:2020-04-10
中文翻译:

星形胶质细胞对炎症的代谢反应受核因子-κB 信号的调节。
炎症和代谢与诱导细胞代谢变化的炎症刺激有着内在的联系,进而决定细胞炎症反应的代谢能力。尽管在外周免疫细胞中得到了很好的表征,但对星形胶质细胞中的这些“免疫代谢”反应知之甚少。在这项研究中,我们检验了由核因子-κB (NF-κB) 信号驱动的星形胶质细胞炎症反应依赖于糖酵解代谢的假设。使用小鼠原代皮质星形胶质细胞培养物,我们评估了暴露于脂多糖 (LPS) 后细胞代谢的变化,并使用细胞因子 ELISA 和免疫印迹来测量炎症反应。结果表明星形胶质细胞对促炎刺激的代谢适应在时间上不同:3 小时 LPS 处理增加了糖酵解,但没有改变线粒体代谢,而在 LPS 处理 24 小时后,我们观察到氧化磷酸化增加,糖酵解能力和葡萄糖摄取降低,部分原因是葡萄糖转运蛋白 1 表达减少。用 IKK-β 抑制剂 TPCA-1 抑制 NF-κB 信号可以防止 LPS 诱导的糖酵解和氧化磷酸化变化。此外,TPCA-1 治疗独立于炎症刺激改变糖酵解和氧化磷酸化,表明 NF-κB 信号在调节星形胶质细胞基础代谢中的作用。用 2-脱氧葡萄糖抑制糖酵解显着减弱了 LPS 诱导的细胞因子释放和 NF-κB 磷酸化,表明对 LPS 的完全炎症反应需要完整的糖酵解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号