当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploring the Chemistry of Spiroindolenines by Mechanistically-Driven Reaction Development: Asymmetric Pictet-Spengler-type Reactions and Beyond.
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2020-04-10 , DOI: 10.1021/acs.accounts.0c00074 Chao Zheng 1 , Shu-Li You 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2020-04-10 , DOI: 10.1021/acs.accounts.0c00074 Chao Zheng 1 , Shu-Li You 1
Affiliation
|
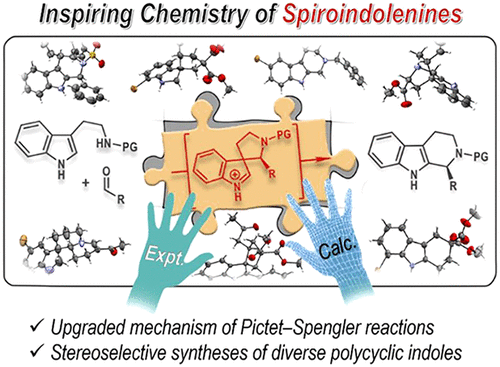
ConspectusThe Pictet-Spengler reaction is a fundamental named reaction in organic chemistry, and it is the most straightforward method for the synthesis of tetrahydro-β-carbolines, a core structure embedded in numerous alkaloids. Spiroindolenines are often proposed as possible intermediates in Pictet-Spengler reactions. However, whether the spiroindolenine species is an intermediate in the mechanism of the asymmetric Pictet-Spengler reaction remains unclear. Questions about the role of the spiroindolenine species regarding the mechanism include the following: Can the spiroindolenine species be formed effectively under Pictet-Spengler conditions? If so, what is its fate? Is the delivery of the enantioenriched tetrahydro-β-carboline product related to the spiroindolenine intermediate? Previous studies regarding these questions have not reached a consensus. Therefore, elucidating these questions will advance the field of synthetic organic chemistry.The first highly enantioselective synthesis of spiroindolenines that have the same molecular scaffold as the proposed key intermediate of the Pictet-Spengler reaction was accomplished by an Ir-catalyzed intramolecular asymmetric allylic substitution reaction of an indol-3-yl allylic carbonate. In this reaction, a piperidine, pyrrolidine, or cyclopentane ring can be introduced in conjunction with the indolenine structure.Spiroindolenines were found to undergo ring-expansive migration reactions when treated with a catalytic amount of an acid, leading to tetrahydro-β-carbolines or related tetrahydrocarbazoles. Comprehensive DFT calculations and Born-Oppenheimer molecular dynamics simulations have provided insight into the mechanism of the migration process. It has been found that the stereochemistry is strongly correlated with the electronic properties of the migratory group along with the acidity of the catalyst. Close interactions between the positively charged migratory group and the electron-rich indole ring favor the stereospecificity of the migration. Furthermore, a continuous mechanistic spectrum of the Pictet-Spengler reactions can be obtained on the basis of two readily accessible energetic parameters that are derived from computed energies for competing transition states relative to a key intermediate species. This theoretical model provides a unified mechanistic understanding of the asymmetric Pictet-Spengler reaction, which has been further supported by rationally designed prototype reactions. Chemically and stereochemically controllable migration can be achieved when multiple potential migratory groups are available.The reactivity of spiroindolenines has also been explored beyond Pictet-Spengler reactions. A one-pot Ir-catalyzed asymmetric allylic dearomatization/stereoconvergent migration allows the facile synthesis of enantioenriched tetrahydro-β-carbolines from racemic starting materials. An unprecedented six- to seven-membered ring-expansive migration can be achieved when a vinyliminium moiety is involved as a highly reactive migratory group. This reaction facilitates the stereoselective synthesis of thermodynamically challenging indole-annulated seven-membered rings. It has also been found that the migration process can be interrupted. The electrophilic migratory group released from the retro-Mannich reaction of a spiroindolenine can be captured by an inter- or intramolecular nucleophile, thus providing new entries into structurally diverse polycyclic indole derivatives.Therefore, the mechanism of the Pictet-Spengler reaction can be probed by manipulating the reactivity of the spiroindolenine species. In turn, the mechanistic insights gained herein will aid in chemical transformations toward various target molecules. This study serves as a vivid example of the positive interplay between experimental and theoretical investigations in synthetic organic chemistry.
中文翻译:

通过机理驱动的反应发展探索螺螺吲哚的化学性质:非对称Pictet-Spengler型反应及以后。
概论Pictet-Spengler反应是有机化学中一个基本的命名反应,它是合成四氢-β-咔啉的最直接方法,四氢-β-咔啉是嵌入许多生物碱中的核心结构。螺环吲哚胺通常被建议作为Pictet-Spengler反应的可能中间体。但是,螺环吲哚胺物种是否是不对称Pictet-Spengler反应机理的中间产物仍不清楚。关于螺环吲哚物质的作用机理的疑问包括:在Pictet-Spengler条件下能否有效形成螺环吲哚物质?如果是这样,它的命运是什么?富含对映体的四氢-β-咔啉产物的提供与螺环吲哚烯中间体有关吗?先前有关这些问题的研究尚未达成共识。因此,阐明这些问题将推动合成有机化学领域的发展。通过Ir催化的分子内不对称烯丙基取代反应,完成了与拟南芥-斯宾格勒反应拟议关键中间体具有相同分子骨架的螺环吲哚胺的首次高对映选择性合成。吲哚-3-基烯丙基碳酸酯的衍生物。在该反应中,可以将哌啶,吡咯烷或环戊烷环与吲哚烯结构结合引入。发现螺环吲哚烯经过催化量的酸处理后会发生扩环迁移反应,导致生成四氢-β-咔啉或相关的四氢咔唑。全面的DFT计算和Born-Oppenheimer分子动力学模拟提供了有关迁移过程机理的见解。已经发现,立体化学与迁移基团的电子性质以及催化剂的酸度密切相关。带正电的迁移基团与富含电子的吲哚环之间的紧密相互作用有利于迁移的立体特异性。此外,可以基于两个容易获得的高能参数来获得Pictet-Spengler反应的连续机械能谱,这是从相对于关键中间物种竞争的过渡态的计算出的能量中得出的。该理论模型提供了对不对称Pictet-Spengler反应的统一机理理解,并得到了合理设计的原型反应的进一步支持。当有多个潜在的迁移基团时,可以实现化学和立体化学可控的迁移。除Pictet-Spengler反应外,还研究了螺环吲哚胺的反应性。一锅Ir催化的不对称烯丙基芳烃脱芳香化/立体收敛迁移使得可以从外消旋起始原料轻松合成对映体富集的四氢β-咔啉。当乙烯基亚胺基部分作为高反应性迁移基团参与时,可以实现前所未有的六元至七元环膨胀迁移。该反应有助于热力学上具有挑战性的吲哚环化的七元环的立体选择性合成。还发现迁移过程可能会中断。螺吲哚烯醛的逆曼尼希反应释放的亲电迁移基团可以被分子间或分子内亲核试剂捕获,从而为结构多样的多环吲哚衍生物提供了新的入口,因此,Pictet-Spengler反应的机理可以通过操纵螺环蛋白烯物种的反应性。继而,本文获得的机械学见解将有助于向各种靶分子的化学转化。这项研究是合成有机化学实验与理论研究之间积极相互作用的生动例证。还发现迁移过程可能会中断。螺吲哚烯醛的逆曼尼希反应释放的亲电迁移基团可以被分子间或分子内亲核试剂捕获,从而为结构多样的多环吲哚衍生物提供了新的入口,因此,Pictet-Spengler反应的机理可以通过操纵螺环蛋白烯物种的反应性。继而,本文获得的机械学见解将有助于向各种靶分子的化学转化。这项研究是合成有机化学实验与理论研究之间积极相互作用的生动例证。还发现迁移过程可能会中断。螺吲哚烯醛的逆曼尼希反应释放的亲电迁移基团可以被分子间或分子内亲核试剂捕获,从而为结构多样的多环吲哚衍生物提供了新的入口,因此,Pictet-Spengler反应的机理可以通过操纵螺环蛋白烯物种的反应性。继而,本文获得的机械学见解将有助于向各种靶分子的化学转化。这项研究是合成有机化学实验与理论研究之间积极相互作用的生动例证。螺吲哚烯醛的逆曼尼希反应释放的亲电迁移基团可以被分子间或分子内亲核试剂捕获,从而为结构多样的多环吲哚衍生物提供了新的入口,因此,Pictet-Spengler反应的机理可以通过操纵螺环蛋白烯物种的反应性。继而,本文获得的机械学见解将有助于向各种靶分子的化学转化。这项研究是合成有机化学实验与理论研究之间积极相互作用的生动例证。螺吲哚烯醛的逆曼尼希反应释放的亲电迁移基团可以被分子间或分子内亲核试剂捕获,从而为结构多样的多环吲哚衍生物提供了新的入口,因此,Pictet-Spengler反应的机理可以通过操纵螺环蛋白烯物种的反应性。继而,本文获得的机械学见解将有助于向各种靶分子的化学转化。这项研究是合成有机化学实验与理论研究之间积极相互作用的生动例证。Pictet-Spengler反应的机理可以通过控制螺环吲哚胺物种的反应性来探究。继而,本文获得的机械学见解将有助于向各种靶分子的化学转化。这项研究是合成有机化学实验与理论研究之间积极相互作用的生动例证。Pictet-Spengler反应的机理可以通过控制螺环吲哚胺物种的反应性来探究。继而,本文获得的机械学见解将有助于向各种靶分子的化学转化。这项研究是合成有机化学实验与理论研究之间积极相互作用的生动例证。
更新日期:2020-04-23
中文翻译:

通过机理驱动的反应发展探索螺螺吲哚的化学性质:非对称Pictet-Spengler型反应及以后。
概论Pictet-Spengler反应是有机化学中一个基本的命名反应,它是合成四氢-β-咔啉的最直接方法,四氢-β-咔啉是嵌入许多生物碱中的核心结构。螺环吲哚胺通常被建议作为Pictet-Spengler反应的可能中间体。但是,螺环吲哚胺物种是否是不对称Pictet-Spengler反应机理的中间产物仍不清楚。关于螺环吲哚物质的作用机理的疑问包括:在Pictet-Spengler条件下能否有效形成螺环吲哚物质?如果是这样,它的命运是什么?富含对映体的四氢-β-咔啉产物的提供与螺环吲哚烯中间体有关吗?先前有关这些问题的研究尚未达成共识。因此,阐明这些问题将推动合成有机化学领域的发展。通过Ir催化的分子内不对称烯丙基取代反应,完成了与拟南芥-斯宾格勒反应拟议关键中间体具有相同分子骨架的螺环吲哚胺的首次高对映选择性合成。吲哚-3-基烯丙基碳酸酯的衍生物。在该反应中,可以将哌啶,吡咯烷或环戊烷环与吲哚烯结构结合引入。发现螺环吲哚烯经过催化量的酸处理后会发生扩环迁移反应,导致生成四氢-β-咔啉或相关的四氢咔唑。全面的DFT计算和Born-Oppenheimer分子动力学模拟提供了有关迁移过程机理的见解。已经发现,立体化学与迁移基团的电子性质以及催化剂的酸度密切相关。带正电的迁移基团与富含电子的吲哚环之间的紧密相互作用有利于迁移的立体特异性。此外,可以基于两个容易获得的高能参数来获得Pictet-Spengler反应的连续机械能谱,这是从相对于关键中间物种竞争的过渡态的计算出的能量中得出的。该理论模型提供了对不对称Pictet-Spengler反应的统一机理理解,并得到了合理设计的原型反应的进一步支持。当有多个潜在的迁移基团时,可以实现化学和立体化学可控的迁移。除Pictet-Spengler反应外,还研究了螺环吲哚胺的反应性。一锅Ir催化的不对称烯丙基芳烃脱芳香化/立体收敛迁移使得可以从外消旋起始原料轻松合成对映体富集的四氢β-咔啉。当乙烯基亚胺基部分作为高反应性迁移基团参与时,可以实现前所未有的六元至七元环膨胀迁移。该反应有助于热力学上具有挑战性的吲哚环化的七元环的立体选择性合成。还发现迁移过程可能会中断。螺吲哚烯醛的逆曼尼希反应释放的亲电迁移基团可以被分子间或分子内亲核试剂捕获,从而为结构多样的多环吲哚衍生物提供了新的入口,因此,Pictet-Spengler反应的机理可以通过操纵螺环蛋白烯物种的反应性。继而,本文获得的机械学见解将有助于向各种靶分子的化学转化。这项研究是合成有机化学实验与理论研究之间积极相互作用的生动例证。还发现迁移过程可能会中断。螺吲哚烯醛的逆曼尼希反应释放的亲电迁移基团可以被分子间或分子内亲核试剂捕获,从而为结构多样的多环吲哚衍生物提供了新的入口,因此,Pictet-Spengler反应的机理可以通过操纵螺环蛋白烯物种的反应性。继而,本文获得的机械学见解将有助于向各种靶分子的化学转化。这项研究是合成有机化学实验与理论研究之间积极相互作用的生动例证。还发现迁移过程可能会中断。螺吲哚烯醛的逆曼尼希反应释放的亲电迁移基团可以被分子间或分子内亲核试剂捕获,从而为结构多样的多环吲哚衍生物提供了新的入口,因此,Pictet-Spengler反应的机理可以通过操纵螺环蛋白烯物种的反应性。继而,本文获得的机械学见解将有助于向各种靶分子的化学转化。这项研究是合成有机化学实验与理论研究之间积极相互作用的生动例证。螺吲哚烯醛的逆曼尼希反应释放的亲电迁移基团可以被分子间或分子内亲核试剂捕获,从而为结构多样的多环吲哚衍生物提供了新的入口,因此,Pictet-Spengler反应的机理可以通过操纵螺环蛋白烯物种的反应性。继而,本文获得的机械学见解将有助于向各种靶分子的化学转化。这项研究是合成有机化学实验与理论研究之间积极相互作用的生动例证。螺吲哚烯醛的逆曼尼希反应释放的亲电迁移基团可以被分子间或分子内亲核试剂捕获,从而为结构多样的多环吲哚衍生物提供了新的入口,因此,Pictet-Spengler反应的机理可以通过操纵螺环蛋白烯物种的反应性。继而,本文获得的机械学见解将有助于向各种靶分子的化学转化。这项研究是合成有机化学实验与理论研究之间积极相互作用的生动例证。Pictet-Spengler反应的机理可以通过控制螺环吲哚胺物种的反应性来探究。继而,本文获得的机械学见解将有助于向各种靶分子的化学转化。这项研究是合成有机化学实验与理论研究之间积极相互作用的生动例证。Pictet-Spengler反应的机理可以通过控制螺环吲哚胺物种的反应性来探究。继而,本文获得的机械学见解将有助于向各种靶分子的化学转化。这项研究是合成有机化学实验与理论研究之间积极相互作用的生动例证。


























 京公网安备 11010802027423号
京公网安备 11010802027423号