当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Evolution of Anion Relay Chemistry: Construction of Architecturally Complex Natural Products.
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2020-04-09 , DOI: 10.1021/acs.accounts.0c00076 Yifan Deng 1 , Amos B Smith 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2020-04-09 , DOI: 10.1021/acs.accounts.0c00076 Yifan Deng 1 , Amos B Smith 1
Affiliation
|
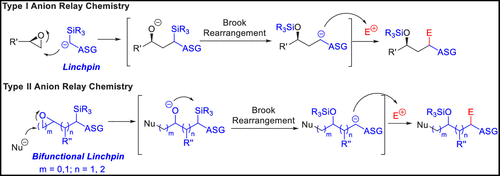
Multicomponent union tactics in which three or more fragments are rapidly connected are highly prized in the construction of architecturally complex natural products. Anion Relay Chemistry (ARC), a multicomponent union tactic, has just such potential to elaborate structurally diverse scaffolds in a single operation with excellent stereochemical control. Conceptually, the ARC tactic can be divided into two main classes: "Through-Bond," by the relay of negative charge through the bonding system of a molecule; and "Through-Space," by the migration of negative charge across space by a transfer agent. "Through-Space" Anion Relay Chemistry, the focus of this Account, can be further subdivided into two types: Type I ARC, originated from the Tietze-Schaumann-Smith coupling reaction, which for the first time permits controllable Brook rearrangements to construct unsymmetrical adducts, and as such has been successfully employed in the total syntheses of diverse natural products, including the mycoticins, bryostatin 1, spongistatins, rimocidin, indolizidine alkaloids, and enigmazole A; and Type II ARC, central to which is the design of novel bifunctional linchpins that enable rapid assembly of linear and cyclic fragments with diverse architectural features, ranging from polyols, spiroketals, and polyenes to polypropionate scaffolds. Recently, the Type II ARC tactic has been exploited as the key construction tactic in the total syntheses of the spirastrellolides, the cryptocarya acetates, secu'amamine A, mandelalide A, and nahuoic acid Ci (Bii). This Account will present the evolution of both the Type I and Type II Anion Relay tactics, in conjunction with some prominent applications.
中文翻译:

阴离子中继化学的演变:建筑复杂的天然产物的构建。
快速连接三个或更多片段的多组件联合策略在建筑复杂的天然产品的构建中倍受青睐。阴离子中继化学(ARC)是一种多组分联合策略,具有这样的潜力,可以在一次操作中通过出色的立体化学控制来制作结构多样的支架。从概念上讲,ARC策略可分为两个主要类别:“穿透键”,通过分子的键合系统传递负电荷;通过分子的键合系统传递负电荷;通过分子间的键合系统传递负电荷。和“穿越空间”,即通过转移剂使负电荷跨空间迁移。这个帐户的重点是“穿越空间”阴离子中继化学,可以进一步分为两种类型:I型ARC,起源于Tietze-Schaumann-Smith偶联反应,首次允许可控的布鲁克重排以构建不对称的加合物,因此已成功用于各种天然产物的总合成中,包括霉菌素,溴抑素1,海绵抑素,rimocidin,吲哚并立定生物碱和依格咪唑A;II型ARC,其中心是新颖的双功能固定销的设计,该销能够快速组装具有多元建筑特征的线性和环状片段,范围从多元醇,螺环酮和多烯到聚丙烯酸酯支架。最近,II型ARC策略已被用作螺旋藻内酯,醋酸隐孢子虫酯,secu'amamine A,mandelalide A和nahuoic acid Ci(Bii)的总合成中的关键构建策略。
更新日期:2020-04-23
中文翻译:

阴离子中继化学的演变:建筑复杂的天然产物的构建。
快速连接三个或更多片段的多组件联合策略在建筑复杂的天然产品的构建中倍受青睐。阴离子中继化学(ARC)是一种多组分联合策略,具有这样的潜力,可以在一次操作中通过出色的立体化学控制来制作结构多样的支架。从概念上讲,ARC策略可分为两个主要类别:“穿透键”,通过分子的键合系统传递负电荷;通过分子的键合系统传递负电荷;通过分子间的键合系统传递负电荷。和“穿越空间”,即通过转移剂使负电荷跨空间迁移。这个帐户的重点是“穿越空间”阴离子中继化学,可以进一步分为两种类型:I型ARC,起源于Tietze-Schaumann-Smith偶联反应,首次允许可控的布鲁克重排以构建不对称的加合物,因此已成功用于各种天然产物的总合成中,包括霉菌素,溴抑素1,海绵抑素,rimocidin,吲哚并立定生物碱和依格咪唑A;II型ARC,其中心是新颖的双功能固定销的设计,该销能够快速组装具有多元建筑特征的线性和环状片段,范围从多元醇,螺环酮和多烯到聚丙烯酸酯支架。最近,II型ARC策略已被用作螺旋藻内酯,醋酸隐孢子虫酯,secu'amamine A,mandelalide A和nahuoic acid Ci(Bii)的总合成中的关键构建策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号