当前位置:
X-MOL 学术
›
Batteries Supercaps
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hollow MoS3 Nanospheres as Electrode Material for “Water‐in‐Salt” Li–Ion Batteries
Batteries & Supercaps ( IF 5.7 ) Pub Date : 2020-04-08 , DOI: 10.1002/batt.202000042 Ting Quan 1 , Yaolin Xu 1 , Michael Tovar 2 , Nicolas Goubard‐Bretesché 3 , Zhaolong Li 4 , Zdravko Kochovski 1 , Holm Kirmse 5 , Kai Skrodczky 6 , Shilin Mei 1 , Hongtao Yu 1 , Daniel Abou‐Ras 2 , Marnix Wagemaker 4 , Yan Lu 1, 7
Batteries & Supercaps ( IF 5.7 ) Pub Date : 2020-04-08 , DOI: 10.1002/batt.202000042 Ting Quan 1 , Yaolin Xu 1 , Michael Tovar 2 , Nicolas Goubard‐Bretesché 3 , Zhaolong Li 4 , Zdravko Kochovski 1 , Holm Kirmse 5 , Kai Skrodczky 6 , Shilin Mei 1 , Hongtao Yu 1 , Daniel Abou‐Ras 2 , Marnix Wagemaker 4 , Yan Lu 1, 7
Affiliation
|
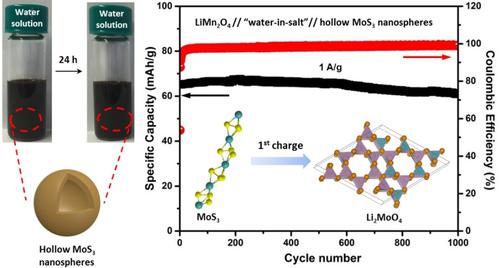
The use of “water‐in‐salt” electrolyte (WISE) (i. e., a highly concentrated aqueous solution) in rechargeable batteries has received increasing attention due to the significantly expanded electrochemical window compared to the limited voltage of conventional aqueous electrolytes. It enables the use of more positive/negative electrode material couples in aqueous batteries, resulting in an enhanced output voltage. However, one of the challenges is to identify promising anode materials for the “water‐in‐salt” Li‐ion batteries (WIS‐LIBs). Herein we for the first time demonstrate that MoS3, an amorphous chain‐like structured transitional metal trichalcogenide, is promising as anode in the WIS‐LIBs. In this work, hollow MoS3 nanospheres were synthesized via a scalable room‐temperature acid precipitation method. When applied in WIS‐LIBs, the prepared MoS3 achieved a high specific capacity of 127 mAh/g at the current density of 0.1 A/g and good stability over 1000 cycles. During operation, MoS3 underwent irreversible conversion to Li2MoO4 (with H2S and H2 evolution) during the initial Li ion uptake, and was then converted gradually to a more stable and reversible Lix MoOy (2≤y ≤4)) phase along cycling. Amorphous Li‐deficient Lix‐m MoOy /MoOz was formed upon delithiation. Nevertheless, MoS3 outperformed MoO3 in WIS‐LIBs, which could be accredited to its initial one‐dimensional molecular structure and the amorphous nature of the delithiated product facilitating charge transport. These results demonstrated a novel routine for synthesizing metal sulfides with hollow structures using a template‐based method and push forward the development of metal sulfides for aqueous energy storage applications.
中文翻译:

空心MoS3纳米球作为“盐包水”锂离子电池的电极材料
由于与常规水性电解质的有限电压相比,电化学窗口显着扩大,因此在可充电电池中使用“盐分水”电解质(WISE)(即高浓度水溶液)受到了越来越多的关注。它可以在水性电池中使用更多的正/负电极材料,从而提高输出电压。但是,挑战之一是为“盐包水”锂离子电池(WIS-LIB)确定有前景的负极材料。在此,我们首次证明MoS 3是一种无定形链状结构过渡金属三卤化钨,有望在WIS-LIB中用作阳极。在这项工作中,空心MoS 3纳米球是通过可扩展的室温酸沉淀法合成的。当用于WIS-LIB中时,制得的MoS 3在0.1 A / g的电流密度下可实现127 mAh / g的高比容量,并在1000次循环中具有良好的稳定性。在操作期间,的MoS 3后行不可逆转换对于Li 2的MoO 4(用H 2 S和H ^ 2放出)的初始的Li离子的摄取期间,然后逐渐转化成更稳定和可逆的锂X的MoO ý(2≤ ÿ ≤ 4))沿循环阶段。非晶锂缺陷型锂X -米的MoO ÿ /的MoOz是在脱锂时形成的。尽管如此,MoS 3在WIS-LIBs中的性能优于MoO 3,这可以归因于其最初的一维分子结构和去锂化产物的无定形性质,从而促进了电荷传输。这些结果证明了使用基于模板的方法合成具有中空结构的金属硫化物的新颖程序,并推动了用于含水能量存储应用的金属硫化物的开发。
更新日期:2020-04-08
中文翻译:

空心MoS3纳米球作为“盐包水”锂离子电池的电极材料
由于与常规水性电解质的有限电压相比,电化学窗口显着扩大,因此在可充电电池中使用“盐分水”电解质(WISE)(即高浓度水溶液)受到了越来越多的关注。它可以在水性电池中使用更多的正/负电极材料,从而提高输出电压。但是,挑战之一是为“盐包水”锂离子电池(WIS-LIB)确定有前景的负极材料。在此,我们首次证明MoS 3是一种无定形链状结构过渡金属三卤化钨,有望在WIS-LIB中用作阳极。在这项工作中,空心MoS 3纳米球是通过可扩展的室温酸沉淀法合成的。当用于WIS-LIB中时,制得的MoS 3在0.1 A / g的电流密度下可实现127 mAh / g的高比容量,并在1000次循环中具有良好的稳定性。在操作期间,的MoS 3后行不可逆转换对于Li 2的MoO 4(用H 2 S和H ^ 2放出)的初始的Li离子的摄取期间,然后逐渐转化成更稳定和可逆的锂X的MoO ý(2≤ ÿ ≤ 4))沿循环阶段。非晶锂缺陷型锂X -米的MoO ÿ /的MoOz是在脱锂时形成的。尽管如此,MoS 3在WIS-LIBs中的性能优于MoO 3,这可以归因于其最初的一维分子结构和去锂化产物的无定形性质,从而促进了电荷传输。这些结果证明了使用基于模板的方法合成具有中空结构的金属硫化物的新颖程序,并推动了用于含水能量存储应用的金属硫化物的开发。



























 京公网安备 11010802027423号
京公网安备 11010802027423号