Applied Catalysis B: Environment and Energy ( IF 22.1 ) Pub Date : 2020-04-08 , DOI: 10.1016/j.apcatb.2020.118949 Shengjie Xia , Lei Fang , Yue Meng , Xueqiang Zhang , Lianyang Zhang , Chao Yang , Zheming Ni
|
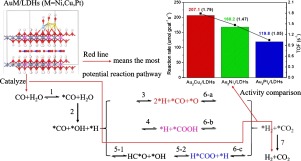
Water-gas shift reaction (WGSR) is an industrialized chemical process with numerous applications in CO removal, H2 generation and coupled in energy storage and reforming reactions involving hydrocarbons, alcohols and Fisher-Tropsch synthesis (FTS). The challenge of WGSR has been the lack of highly active and stable catalyst at low operational temperatures because conventional Cu-Zn and Co-Mo based catalysts suffer quick activity loss under working conditions. Au and AuM (M=Ni, Cu, Pt) alloy nanoparticles supported on layered double hydroxides (LDHs) were prepared and characterized in terms of their structural, morphological and chemical properties. It was found that the incorporation of Au significantly enhances the catalytic activity of LDHs for WGSR at temperatures of practical catalytic relevance (450–550 K) and the performance can be further engineered via tuning the geometrical environment of Au by alloying with a 2nd metal (Ni, Cu and Pt). Temperature programmed reduction (TPR) and Au dispersion experiments suggest that the addition of AuM modulates the redox circle at the metal/LDHs interface with Au2Cu1 yielding the highest turnover frequency (TOF). In-situ DRIFTS captures the evolution of surface reactive species and suggests a reaction pathway via the formation of formate (HCOO*). While the formate route dominates the AuM/LDHs catalyzed WGSR, the redox mechanism can also be activated by bypassing a direct *O-H bond breakage step that requires prohibitively high activation energy. Consistent results were obtained in our DFT calculations, where the AuM/LDHs catalysts were found facilitating the WGSR reaction by preferentially mediating a formate pathway. Our combinative theoretical and experimental study suggests that LDHs is a family of promising low-cost, stable and highly active supporting materials for practical heterogeneous catalysis and demonstrates a strategic way to understand and engineer the fundamentals of a reaction that benefits the whole chemical transformation.
中文翻译:

层状双氢氧化物负载的Au-Ni / Cu / Pt双金属合金催化的水煤气变换反应
水煤气变换反应(WGSR)是工业化的化学过程,在CO去除,H 2去除方面有许多应用产生并耦合到涉及碳氢化合物,醇和费托合成(FTS)的能量存储和重整反应中。WGSR的挑战在于在低操作温度下缺乏高活性和稳定的催化剂,因为常规的基于Cu-Zn和Co-Mo的催化剂在工作条件下会很快失去活性。制备了负载在层状双氢氧化物(LDHs)上的Au和AuM(M = Ni,Cu,Pt)合金纳米颗粒,并根据其结构,形态和化学性质对其进行了表征。发现在实际催化相关温度(450–550 K)的温度下,掺入Au可以显着增强LDHs对WGSR的催化活性,并且可以通过与第二种金属合金化来调节Au的几何环境,从而进一步设计性能。 Ni,Cu和Pt)。2铜1产生最高的周转频率(TOF)。原位DRIFTS捕获表面反应性物种的演变,并暗示通过甲酸(HCOO *)形成的反应途径。尽管甲酸盐途径在AuM / LDHs催化的WGSR中占主导地位,但还可以通过绕过直接的* OH键断裂步骤来激活氧化还原机理,该步骤需要极高的活化能。在我们的DFT计算中获得了一致的结果,其中发现AuM / LDHs催化剂通过优先介导甲酸途径而促进WGSR反应。我们的综合理论和实验研究表明,LDH是一个有前途的低成本家族,



























 京公网安备 11010802027423号
京公网安备 11010802027423号