当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Experimental Study and Modeling of Bubble Point of Aqueous Mixtures of Deep Eutectic Solvents Based on Dicarboxylic Acids and Choline Chloride
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-04-08 , DOI: 10.1021/acs.jced.0c00070 Sahar Gholami 1 , Aliakbar Roosta 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-04-08 , DOI: 10.1021/acs.jced.0c00070 Sahar Gholami 1 , Aliakbar Roosta 1
Affiliation
|
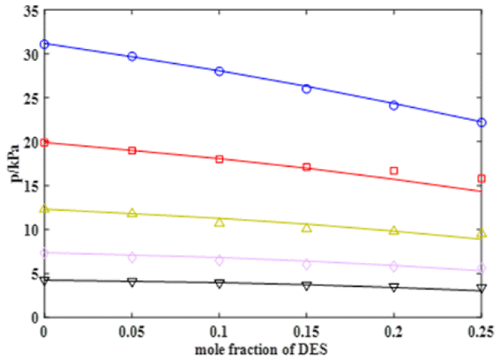
Assessment of deep eutectic solvents (DESs) as a new generation of green solvent for different applications requires sufficient knowledge about their physical, chemical, and thermodynamic properties. Because of the lack of bubble pressure data of aqueous solutions of DESs, the bubble pressure of two natural DESs, choline chloride–malic acid and choline chloride–malonic acid, as well as the bubble pressure of malic acid–water and malonic acid–water, was measured at various concentrations and at a temperature range from 303.2 to 343.2 K. According to the results, all studied systems exhibit a negative deviation from Raoult’s law. The order of the negative deviation was malic acid > malonic acid > DES of choline chloride–malic acid > DES of choline chloride–malonic acid. The experimental bubble points were used for estimation of the water activity coefficient and consequently for estimation of the parameters of the nonrandom two-liquid (NRTL) and van Laar models. Then, the models were employed to estimate the bubble point of the studied systems. According to the results, the overall average absolute relative deviation of the NRTL and van Laar models were obtained to be 2.3% and 2.4%, respectively, which shows that the experimental data correlate well with the models’ results.
中文翻译:

基于二羧酸和氯化胆碱的深共熔溶剂水混合物沸点的实验研究和建模
评估深共熔溶剂(DESs)作为适用于不同应用的新一代绿色溶剂需要对它们的物理,化学和热力学性质有足够的了解。由于缺乏DES水溶液的气泡压力数据,因此两种天然DES的气泡压力为氯化胆碱-苹果酸和胆碱氯化物-丙二酸,以及苹果酸-水和丙二酸-水的气泡压力。在各种浓度下,在303.2至343.2 K的温度范围内测量γ。根据结果,所有研究的系统均表现出与拉乌尔定律的负偏差。负偏差的顺序为苹果酸>丙二酸>氯化胆碱-苹果酸的DES>氯化胆碱-丙二酸的DES。实验起泡点用于估算水活度系数,因此用于估算非随机二液(NRTL)模型和van Laar模型的参数。然后,使用模型来估计研究系统的冒泡点。根据结果,NRTL模型和van Laar模型的总体平均绝对相对偏差分别为2.3%和2.4%,这表明实验数据与模型结果具有很好的相关性。
更新日期:2020-04-08
中文翻译:

基于二羧酸和氯化胆碱的深共熔溶剂水混合物沸点的实验研究和建模
评估深共熔溶剂(DESs)作为适用于不同应用的新一代绿色溶剂需要对它们的物理,化学和热力学性质有足够的了解。由于缺乏DES水溶液的气泡压力数据,因此两种天然DES的气泡压力为氯化胆碱-苹果酸和胆碱氯化物-丙二酸,以及苹果酸-水和丙二酸-水的气泡压力。在各种浓度下,在303.2至343.2 K的温度范围内测量γ。根据结果,所有研究的系统均表现出与拉乌尔定律的负偏差。负偏差的顺序为苹果酸>丙二酸>氯化胆碱-苹果酸的DES>氯化胆碱-丙二酸的DES。实验起泡点用于估算水活度系数,因此用于估算非随机二液(NRTL)模型和van Laar模型的参数。然后,使用模型来估计研究系统的冒泡点。根据结果,NRTL模型和van Laar模型的总体平均绝对相对偏差分别为2.3%和2.4%,这表明实验数据与模型结果具有很好的相关性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号