当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interpreting In Vitro Release Performance from Long-Acting Parenteral Nanosuspensions Using USP-4 Dissolution and Spectroscopic Techniques.
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-04-08 , DOI: 10.1021/acs.molpharmaceut.0c00208 Nathan D Rudd 1 , Mikhail Reibarkh 2 , Rui Fang 3 , Sachin Mittal 3 , Paul L Walsh 1 , Andrew P J Brunskill 1 , William P Forrest 3
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-04-08 , DOI: 10.1021/acs.molpharmaceut.0c00208 Nathan D Rudd 1 , Mikhail Reibarkh 2 , Rui Fang 3 , Sachin Mittal 3 , Paul L Walsh 1 , Andrew P J Brunskill 1 , William P Forrest 3
Affiliation
|
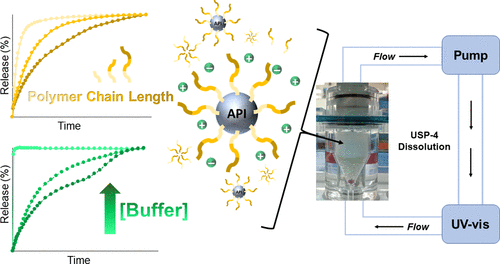
Injectable sustained release dosage forms have emerged as desirable therapeutic routes for patients that require life-long treatments. The prevalence of drug molecules with low aqueous solubility and bioavailability has added momentum toward the development of suspension-based long-acting parenteral (LAP) formulations; the previously undesirable physicochemical properties of Biopharmaceutics Classification System (BCS) Class II/IV compounds are best suited for extended release applications. Effective in vitro release (IVR) testing of crystalline suspensions affirms product quality during early-stage development and provides connections with in vivo performance. However, before in vitro-in vivo correlations (IVIVCs) can be established, it is necessary to evaluate formulation attributes that directly affect IVR properties. In this work, a series of crystalline LAP nanosuspensions were formulated with different stabilizing polymers and applied to a continuous flow-through (USP-4) dissolution method. This technique confirmed the role of salt effects on the stability of polymer-coated nanoparticles through the detection of disparate active pharmaceutical ingredient (API) release profiles. The polymer stabilizers with extended hydrophilic chains exhibited elevated intrapolymer activity from the loss of hydrogen-bond cushioning in dissolution media with heightened ionic strength, confirmed through one-dimensional (1D) 1H NMR and two-dimensional nuclear Overhauser effect spectroscopy (2D NOESY) experiments. Thus, steric repulsion within the affected nanosuspensions was limited and release rates decreased. Additionally, the strength of interaction between hydrophobic polymer components and the API crystalline surface contributed to suspension dissolution properties, confirmed through solution- and solid-state spectroscopic analyses. This study provides a unique perspective on the dynamic interface between the crystalline drug and aqueous microenvironment during dissolution.
中文翻译:

使用USP-4溶出度和光谱技术解释长效肠胃外纳米悬浮液的体外释放性能。
对于需要终生治疗的患者而言,可注射的持续释放剂型已成为理想的治疗途径。具有低水溶性和生物利用度的药物分子的普及为基于悬浮的长效肠胃外(LAP)制剂的开发增加了动力。生物制药分类系统(BCS)II / IV类化合物以前不希望有的理化性质最适合于缓释应用。晶体悬浮液的有效体外释放(IVR)测试确认了早期开发过程中的产品质量,并提供了与体内性能的联系。但是,在可以建立体外-体内相关性(IVIVC)之前,必须评估直接影响IVR特性的制剂属性。在这项工作中 用不同的稳定化聚合物配制了一系列结晶性LAP纳米悬浮液,并应用于连续流通(USP-4)溶解方法。通过检测不同的活性药物成分(API)释放曲线,该技术证实了盐对聚合物包覆的纳米颗粒稳定性的影响。一维(1D)1H NMR和二维核Overhauser效应谱(2D NOESY)实验证实,具有扩展的亲水链的聚合物稳定剂由于溶解介质中氢键缓冲的丧失而具有较高的离子强度,从而提高了聚合物内活性。 。因此,受影响的纳米悬浮液内的空间排斥受到限制,释放速率降低。另外,通过溶液和固态光谱分析证实,疏水性聚合物组分与API晶体表面之间相互作用的强度有助于悬浮液的溶解性能。这项研究为溶解过程中结晶药物与水性微环境之间的动态界面提供了独特的视角。
更新日期:2020-04-08
中文翻译:

使用USP-4溶出度和光谱技术解释长效肠胃外纳米悬浮液的体外释放性能。
对于需要终生治疗的患者而言,可注射的持续释放剂型已成为理想的治疗途径。具有低水溶性和生物利用度的药物分子的普及为基于悬浮的长效肠胃外(LAP)制剂的开发增加了动力。生物制药分类系统(BCS)II / IV类化合物以前不希望有的理化性质最适合于缓释应用。晶体悬浮液的有效体外释放(IVR)测试确认了早期开发过程中的产品质量,并提供了与体内性能的联系。但是,在可以建立体外-体内相关性(IVIVC)之前,必须评估直接影响IVR特性的制剂属性。在这项工作中 用不同的稳定化聚合物配制了一系列结晶性LAP纳米悬浮液,并应用于连续流通(USP-4)溶解方法。通过检测不同的活性药物成分(API)释放曲线,该技术证实了盐对聚合物包覆的纳米颗粒稳定性的影响。一维(1D)1H NMR和二维核Overhauser效应谱(2D NOESY)实验证实,具有扩展的亲水链的聚合物稳定剂由于溶解介质中氢键缓冲的丧失而具有较高的离子强度,从而提高了聚合物内活性。 。因此,受影响的纳米悬浮液内的空间排斥受到限制,释放速率降低。另外,通过溶液和固态光谱分析证实,疏水性聚合物组分与API晶体表面之间相互作用的强度有助于悬浮液的溶解性能。这项研究为溶解过程中结晶药物与水性微环境之间的动态界面提供了独特的视角。


























 京公网安备 11010802027423号
京公网安备 11010802027423号