当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Endothelial cells derived from patients' induced pluripotent stem cells for sustained factor VIII delivery and the treatment of hemophilia A.
STEM CELLS Translational Medicine ( IF 6 ) Pub Date : 2020-03-12 , DOI: 10.1002/sctm.19-0261 Melanie Rose 1 , Kewa Gao 2, 3 , Elizabeth Cortez-Toledo 1 , Emmanuel Agu 1 , Alicia A Hyllen 1 , Kelsey Conroy 1 , Guangjin Pan 4 , Jan A Nolta 1, 5 , Aijun Wang 2, 3, 6 , Ping Zhou 1, 5
STEM CELLS Translational Medicine ( IF 6 ) Pub Date : 2020-03-12 , DOI: 10.1002/sctm.19-0261 Melanie Rose 1 , Kewa Gao 2, 3 , Elizabeth Cortez-Toledo 1 , Emmanuel Agu 1 , Alicia A Hyllen 1 , Kelsey Conroy 1 , Guangjin Pan 4 , Jan A Nolta 1, 5 , Aijun Wang 2, 3, 6 , Ping Zhou 1, 5
Affiliation
|
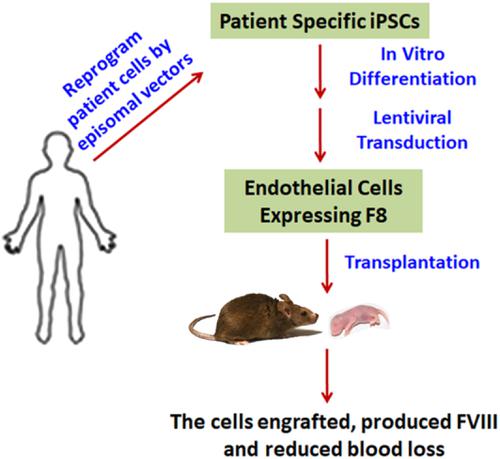
Hemophilia A (HA) is a bleeding disorder characterized by spontaneous and prolonged hemorrhage. The disease is caused by mutations in the coagulation factor 8 gene (F8) leading to factor VIII (FVIII) deficiency. Since FVIII is primarily produced in endothelial cells (ECs) in a non‐diseased human being, ECs hold great potential for development as a cell therapy for HA. We showed that HA patient‐specific induced pluripotent stem cells (HA‐iPSCs) could provide a renewable supply of ECs. The HA‐iPSC‐derived ECs were transduced with lentiviral vectors to stably express the functional B domain deleted F8 gene, the luciferase gene, and the enhanced green fluorescent protein gene (GFP). When transplanted intramuscularly into neonatal and adult immune deficient mice, the HA‐iPSC‐derived ECs were retained in the animals for at least 10‐16 weeks and maintained their expression of FVIII, GFP, and the endothelial marker CD31, as demonstrated by bioluminescence imaging and immunostaining, respectively. When transplanted into HA mice, these transduced HA‐iPSC‐derived ECs significantly reduced blood loss in a tail‐clip bleeding test and produced therapeutic plasma levels (11.2%‐369.2%) of FVIII. Thus, our studies provide proof‐of‐concept that HA‐iPSC‐derived ECs can serve as a factory to deliver FVIII for the treatment of HA not only in adults but also in newborns.
中文翻译:

源自患者诱导的多能干细胞的内皮细胞用于持续VIII因子递送和A型血友病的治疗。
甲型血友病(HA)是一种以自发性和长期出血为特征的出血性疾病。该疾病是由凝血因子8基因(F8)突变导致的凝血因子VIII(FVIII)缺乏引起的。由于FVIII主要是在非疾病人的内皮细胞(EC)中产生的,因此EC具有作为HA细胞疗法的巨大发展潜力。我们表明,HA患者特异性诱导的多能干细胞(HA-iPSC)可以提供可再生的EC。用慢病毒载体转导HA-iPSC衍生的EC,以稳定表达功能性B结构域缺失的F8基因,荧光素酶基因和增强的绿色荧光蛋白基因(GFP)。当通过肌内移植到新生和成年免疫缺陷小鼠中时,HA‐iPSC衍生的EC在动物中保留至少10-16周,并保持FVIII,GFP和内皮标记物CD31的表达,如生物发光成像所证明和免疫染色。将这些转导的HA-iPSC来源的EC移植到HA小鼠中后,在尾夹出血试验中可显着减少失血,并产生FVIII的治疗性血浆水平(11.2%-369.2%)。因此,我们的研究提供了概念证明,即源自HA-iPSC的EC可以作为提供FVIII的工厂,不仅在成人中而且在新生儿中都可以治疗HA。
更新日期:2020-03-12
中文翻译:

源自患者诱导的多能干细胞的内皮细胞用于持续VIII因子递送和A型血友病的治疗。
甲型血友病(HA)是一种以自发性和长期出血为特征的出血性疾病。该疾病是由凝血因子8基因(F8)突变导致的凝血因子VIII(FVIII)缺乏引起的。由于FVIII主要是在非疾病人的内皮细胞(EC)中产生的,因此EC具有作为HA细胞疗法的巨大发展潜力。我们表明,HA患者特异性诱导的多能干细胞(HA-iPSC)可以提供可再生的EC。用慢病毒载体转导HA-iPSC衍生的EC,以稳定表达功能性B结构域缺失的F8基因,荧光素酶基因和增强的绿色荧光蛋白基因(GFP)。当通过肌内移植到新生和成年免疫缺陷小鼠中时,HA‐iPSC衍生的EC在动物中保留至少10-16周,并保持FVIII,GFP和内皮标记物CD31的表达,如生物发光成像所证明和免疫染色。将这些转导的HA-iPSC来源的EC移植到HA小鼠中后,在尾夹出血试验中可显着减少失血,并产生FVIII的治疗性血浆水平(11.2%-369.2%)。因此,我们的研究提供了概念证明,即源自HA-iPSC的EC可以作为提供FVIII的工厂,不仅在成人中而且在新生儿中都可以治疗HA。


























 京公网安备 11010802027423号
京公网安备 11010802027423号