当前位置:
X-MOL 学术
›
J. Neurosci. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Purinergic modulation of neuronal gap junction circuits in the CNS of the leech.
Journal of Neuroscience Research ( IF 4.2 ) Pub Date : 2020-02-25 , DOI: 10.1002/jnr.24599 Oliva Mota Segura 1 , Lina Abdulnoor 1 , Vinh-Vincent Hua 1 , Martha J Solano 1 , Eduardo R Macagno 1 , Michael W Baker 1, 2
Journal of Neuroscience Research ( IF 4.2 ) Pub Date : 2020-02-25 , DOI: 10.1002/jnr.24599 Oliva Mota Segura 1 , Lina Abdulnoor 1 , Vinh-Vincent Hua 1 , Martha J Solano 1 , Eduardo R Macagno 1 , Michael W Baker 1, 2
Affiliation
|
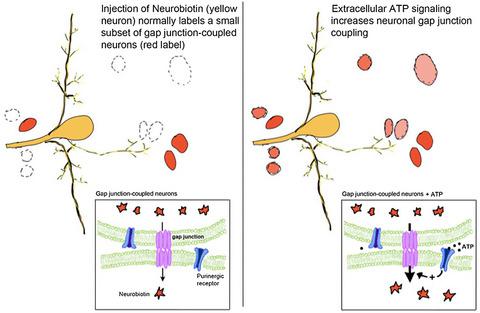
Gap junctions (GJs) are widely distributed in brains across the animal kingdom. To visualize the GJ- coupled networks of two major mechanosensory neurons in the ganglia of medicinal leeches, we injected these cells with the GJ-permeable tracer Neurobiotin. When diffusion time was limited to only 30 min, tracer coupling was highly variable for both cells, suggesting a possible modulation of GJ permeability. In invertebrates the innexins (homologs of vertebrate pannexins) form the GJs. Because extracellular adenosine triphosphate (ATP) modulates pannexin and leech innexin hemichannel permeability and is released by leech glial cells following injury, we tested the effects of bath application of ATP after the injection of Neurobiotin and observed a significant increase in the number of neurons tracer coupled to the sensory neurons. This effect required the elevation of intracellular Ca2+ and could be produced by bath application of caffeine. Conversely, scavenging endogenous extracellular ATP with the ATPase apyrase decreased the number of coupled cells. ATP also increased electrical conductance and tracer permeability between the bilateral Retzius neurons. This modulatory effect of ATP on GJ coupling was blocked by siRNA knockdown of a P1-like adenosine receptor. Finally, exposure of leech ganglia to extracellular ATP induced a characteristic low frequency (<0.3 Hz) rhythmic bursting activity that was roughly synchronous among multiple neurons, a behavior that was significantly attenuated by the GJ blocker octanol. These findings highlight the mediation by ATP of a robust physiological mechanism for modifying neuronal circuits by rapidly recruiting neurons into active networks and entraining synchronized bursting activity.
中文翻译:

水蛭中枢神经系统中神经元间隙连接回路的嘌呤能调节。
间隙连接 (GJ) 广泛分布在整个动物界的大脑中。为了可视化药用水蛭神经节中两个主要机械感觉神经元的 GJ 耦合网络,我们向这些细胞注射了 GJ 可渗透示踪剂神经生物素。当扩散时间仅限于 30 分钟时,两种细胞的示踪剂耦合变化很大,表明 GJ 渗透性可能发生调节。在无脊椎动物中,innexins(脊椎动物 pannexins 的同系物)形成 GJ。因为细胞外三磷酸腺苷 (ATP) 调节 pannexin 和水蛭 innexin 半通道通透性,并在受伤后由水蛭神经胶质细胞释放,我们测试了注射神经生物素后沐浴应用 ATP 的效果,并观察到耦合的神经元示踪剂数量显着增加到感觉神经元。这种效果需要细胞内 Ca2+ 的升高,并且可以通过沐浴应用咖啡因产生。相反,用 ATPase apyrase 清除内源性细胞外 ATP 会减少偶联细胞的数量。ATP 还增加了双侧 Retzius 神经元之间的电导和示踪剂渗透性。ATP 对 GJ 偶联的这种调节作用被 P1 样腺苷受体的 siRNA 组合式阻断。最后,将水蛭神经节暴露于细胞外 ATP 会诱导特征性的低频 (<0.3 Hz) 节律性爆发活动,该活动在多个神经元之间大致同步,这种行为被 GJ 阻滞剂辛醇显着减弱。
更新日期:2020-02-25
中文翻译:

水蛭中枢神经系统中神经元间隙连接回路的嘌呤能调节。
间隙连接 (GJ) 广泛分布在整个动物界的大脑中。为了可视化药用水蛭神经节中两个主要机械感觉神经元的 GJ 耦合网络,我们向这些细胞注射了 GJ 可渗透示踪剂神经生物素。当扩散时间仅限于 30 分钟时,两种细胞的示踪剂耦合变化很大,表明 GJ 渗透性可能发生调节。在无脊椎动物中,innexins(脊椎动物 pannexins 的同系物)形成 GJ。因为细胞外三磷酸腺苷 (ATP) 调节 pannexin 和水蛭 innexin 半通道通透性,并在受伤后由水蛭神经胶质细胞释放,我们测试了注射神经生物素后沐浴应用 ATP 的效果,并观察到耦合的神经元示踪剂数量显着增加到感觉神经元。这种效果需要细胞内 Ca2+ 的升高,并且可以通过沐浴应用咖啡因产生。相反,用 ATPase apyrase 清除内源性细胞外 ATP 会减少偶联细胞的数量。ATP 还增加了双侧 Retzius 神经元之间的电导和示踪剂渗透性。ATP 对 GJ 偶联的这种调节作用被 P1 样腺苷受体的 siRNA 组合式阻断。最后,将水蛭神经节暴露于细胞外 ATP 会诱导特征性的低频 (<0.3 Hz) 节律性爆发活动,该活动在多个神经元之间大致同步,这种行为被 GJ 阻滞剂辛醇显着减弱。


























 京公网安备 11010802027423号
京公网安备 11010802027423号