当前位置:
X-MOL 学术
›
J. Surfactants Deterg.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Elucidation of Interactions between l‐Ascorbic Acid and Mixed Micellar Aggregates of Catanionic {Sodium Dodecylsulfate + Cetyltrimethylammonium Bromide} Surfactants via Physicochemical and Spectroscopic Studies
Journal of Surfactants and Detergents ( IF 1.6 ) Pub Date : 2019-08-05 , DOI: 10.1002/jsde.12328 Parampaul K. Banipal 1 , Pallavi Sohal 1 , Sonika Arti 1 , Tarlok S. Banipal 1
Journal of Surfactants and Detergents ( IF 1.6 ) Pub Date : 2019-08-05 , DOI: 10.1002/jsde.12328 Parampaul K. Banipal 1 , Pallavi Sohal 1 , Sonika Arti 1 , Tarlok S. Banipal 1
Affiliation
|
Surfactant micelles mimic the microenvironment present in biological systems and can act as a medium for antioxidant studies. Moreover, the thermodynamic profile of micellization and spectroscopic studies provides very good information about interactions in these systems. Thus, the mixed micellar behavior of sodium dodecylsulfate (SDS) and cetyltrimethylammonium bromide (CTAB) at varying mole fractions of SDS was studied in (0.01, 0.02, and 0.03) mol kg−1 ʟ‐ascorbic acid(aq) solutions with the aid of various techniques viz., conductivity, density and sound velocity, and spectroscopy. From the CMC values of the mixed surfactants, the degree of ionization (β) and thermodynamic parameters ( ,
,  , and
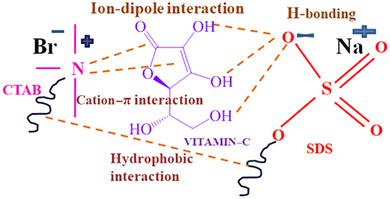
, and  ) were evaluated at 298.15, 308.15, and 318.15 K. The UV absorption spectra were recorded in (1–3) × 10−4 mol kg−1 ʟ‐ascorbic acid(aq) solutions at various mole fractions of SDS. The proton (1H) NMR spectra of mixed (SDS + CTAB) surfactants were studied in (0.01–0.03) mol kg−1 ʟ‐ascorbic acid solutions. Hydrodynamic diameters (Dh) of mixed micellar aggregates were obtained from the dynamic light scattering (DLS) studies. The present studies suggest the predominance of ionic‐hydrophilic interactions between the ionic head groups {O‐SO3− or N+ (CH3)3} of surfactants and the polar (–OH, –C=O and –O–) sites of ʟ‐ascorbic acid.
) were evaluated at 298.15, 308.15, and 318.15 K. The UV absorption spectra were recorded in (1–3) × 10−4 mol kg−1 ʟ‐ascorbic acid(aq) solutions at various mole fractions of SDS. The proton (1H) NMR spectra of mixed (SDS + CTAB) surfactants were studied in (0.01–0.03) mol kg−1 ʟ‐ascorbic acid solutions. Hydrodynamic diameters (Dh) of mixed micellar aggregates were obtained from the dynamic light scattering (DLS) studies. The present studies suggest the predominance of ionic‐hydrophilic interactions between the ionic head groups {O‐SO3− or N+ (CH3)3} of surfactants and the polar (–OH, –C=O and –O–) sites of ʟ‐ascorbic acid.
中文翻译:

通过物理化学和光谱研究阐明了l-抗坏血酸与阳离子{十二烷基硫酸钠+十六烷基三甲基溴化铵}表面活性剂的混合胶束聚集体之间的相互作用
表面活性剂胶束模拟生物系统中存在的微环境,可以作为抗氧化剂研究的介质。此外,胶束化和光谱学研究的热力学概况提供了有关这些系统中相互作用的很好的信息。因此,十二烷基硫酸钠(SDS)在不同的SDS的摩尔分数的混合胶束的行为和十六烷基三甲基铵(CTAB)的(0.01,0.02,和0.03)摩尔kg的研究-1 ʟ抗坏血酸(水溶液)与所述辅助的解决方案的各种技术即,电导率,密度和声速,和光谱。从混合的表面活性剂,离子化(度的CMC值β)和热力学参数( ,
, ,和
,和 )298.15进行了评价,308.15,318.15和K的UV吸收光谱记录在(1-3)×10 -4摩尔千克-1 ʟ抗坏血酸(水溶液)在SDS的各个摩尔分数的解决方案。质子(1 1H)NMR混合(SDS + CTAB)的表面活性剂的光谱中(0.01-0.03)摩尔公斤研究-1 ʟ抗坏血酸溶液。从动态光散射(DLS)研究获得了混合胶束聚集体的流体力学直径(D h)。本研究表明,这种离子头部基团{之间的离子的亲水性相互作用的优势O形SO 3 -或N +(CH 3)3}表面活性剂和ʟ-抗坏血酸的极性位置(-OH,-C = O和-O-)。
)298.15进行了评价,308.15,318.15和K的UV吸收光谱记录在(1-3)×10 -4摩尔千克-1 ʟ抗坏血酸(水溶液)在SDS的各个摩尔分数的解决方案。质子(1 1H)NMR混合(SDS + CTAB)的表面活性剂的光谱中(0.01-0.03)摩尔公斤研究-1 ʟ抗坏血酸溶液。从动态光散射(DLS)研究获得了混合胶束聚集体的流体力学直径(D h)。本研究表明,这种离子头部基团{之间的离子的亲水性相互作用的优势O形SO 3 -或N +(CH 3)3}表面活性剂和ʟ-抗坏血酸的极性位置(-OH,-C = O和-O-)。
更新日期:2019-08-05
 ,
,  , and
, and  ) were evaluated at 298.15, 308.15, and 318.15 K. The UV absorption spectra were recorded in (1–3) × 10−4 mol kg−1 ʟ‐ascorbic acid(aq) solutions at various mole fractions of SDS. The proton (1H) NMR spectra of mixed (SDS + CTAB) surfactants were studied in (0.01–0.03) mol kg−1 ʟ‐ascorbic acid solutions. Hydrodynamic diameters (Dh) of mixed micellar aggregates were obtained from the dynamic light scattering (DLS) studies. The present studies suggest the predominance of ionic‐hydrophilic interactions between the ionic head groups {O‐SO3− or N+ (CH3)3} of surfactants and the polar (–OH, –C=O and –O–) sites of ʟ‐ascorbic acid.
) were evaluated at 298.15, 308.15, and 318.15 K. The UV absorption spectra were recorded in (1–3) × 10−4 mol kg−1 ʟ‐ascorbic acid(aq) solutions at various mole fractions of SDS. The proton (1H) NMR spectra of mixed (SDS + CTAB) surfactants were studied in (0.01–0.03) mol kg−1 ʟ‐ascorbic acid solutions. Hydrodynamic diameters (Dh) of mixed micellar aggregates were obtained from the dynamic light scattering (DLS) studies. The present studies suggest the predominance of ionic‐hydrophilic interactions between the ionic head groups {O‐SO3− or N+ (CH3)3} of surfactants and the polar (–OH, –C=O and –O–) sites of ʟ‐ascorbic acid.
中文翻译:

通过物理化学和光谱研究阐明了l-抗坏血酸与阳离子{十二烷基硫酸钠+十六烷基三甲基溴化铵}表面活性剂的混合胶束聚集体之间的相互作用
表面活性剂胶束模拟生物系统中存在的微环境,可以作为抗氧化剂研究的介质。此外,胶束化和光谱学研究的热力学概况提供了有关这些系统中相互作用的很好的信息。因此,十二烷基硫酸钠(SDS)在不同的SDS的摩尔分数的混合胶束的行为和十六烷基三甲基铵(CTAB)的(0.01,0.02,和0.03)摩尔kg的研究-1 ʟ抗坏血酸(水溶液)与所述辅助的解决方案的各种技术即,电导率,密度和声速,和光谱。从混合的表面活性剂,离子化(度的CMC值β)和热力学参数(
 ,
, ,和
,和 )298.15进行了评价,308.15,318.15和K的UV吸收光谱记录在(1-3)×10 -4摩尔千克-1 ʟ抗坏血酸(水溶液)在SDS的各个摩尔分数的解决方案。质子(1 1H)NMR混合(SDS + CTAB)的表面活性剂的光谱中(0.01-0.03)摩尔公斤研究-1 ʟ抗坏血酸溶液。从动态光散射(DLS)研究获得了混合胶束聚集体的流体力学直径(D h)。本研究表明,这种离子头部基团{之间的离子的亲水性相互作用的优势O形SO 3 -或N +(CH 3)3}表面活性剂和ʟ-抗坏血酸的极性位置(-OH,-C = O和-O-)。
)298.15进行了评价,308.15,318.15和K的UV吸收光谱记录在(1-3)×10 -4摩尔千克-1 ʟ抗坏血酸(水溶液)在SDS的各个摩尔分数的解决方案。质子(1 1H)NMR混合(SDS + CTAB)的表面活性剂的光谱中(0.01-0.03)摩尔公斤研究-1 ʟ抗坏血酸溶液。从动态光散射(DLS)研究获得了混合胶束聚集体的流体力学直径(D h)。本研究表明,这种离子头部基团{之间的离子的亲水性相互作用的优势O形SO 3 -或N +(CH 3)3}表面活性剂和ʟ-抗坏血酸的极性位置(-OH,-C = O和-O-)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号